21 Sep 2021
An Introduction to Imtakt HPLC Columns
By Josep Serret
Imtakt is not the first brand that comes to mind when considering HPLC columns. It is not yet a household name in the U.K., but it enjoys a distinguished reputation in its native Japan and APCA countries. Google Scholar lists over 2300 entries that reference Imtakt. Elsevier/ScienceDirect hosts 500 research articles with Imtakt columns, 200 in Springer, 30 are found in RSC journals, etc.
If any of us have used Imtakt columns, it has been thanks to Simon Lambert, of ARC Sciences, who distributed the brand in the UK. After Simon took his retirement in April, Element picked up the baton. Imtakt Corporation was founded by Dr Itaru Yazawa in 1999. At a time when mainstream HPLC columns used almost exclusively 5μm, porous silica particles. Dr Yazawa started to tour chromatography symposia and trade conventions, talking about the high throughput and efficiency achieved by his newly developed 3μm particle HPLC columns.
People who know the country well have told me that Japanese HPLC manufacturers rely far less on marketing than their western counterparts. It is their belief that the quality of their products should do the talking, and word of mouth should be the best advertisement. If that’s the case, then Dr Yazawa’s columns must have been very eloquent, because by January 2000 Imtakt had launched its first commercial product.
There are six families of columns in the Imtakt catalogue:
- Cadenza, the original range of C18 stationary phases including the first commercially released phase, Cadenza CD-C18.
- Unison, the second family to be launched, including C18, C8, C1, phenyl, amino, and underivatised silica.
- Intrada, optimised for the analysis of biomolecules (Intrada WP-RP (300Å pore size) and Intrada SEC, for protein analysis), amino acids, and organic acids.
- Scherzo, a range of multimode RP/SAX/SCX phases capable of separating neutral, anionic and cationic species under LC-MS compatible conditions.
- Dacapo DX-C18, a 2.5μm “Dual Matrix” porous silica particle, with an organic polymer surface layer designed for extended use at high pH.
Cadenza
A characteristic of Cadenza CD-C18 that quickly stood out was efficiency: the 3μm particle columns could generate comparable theoretical plates to sub-2μm columns, but with much lower backpressures, compatible with traditional HPLC equipment. This is the result of carefully engineered monodispersed silica particles. Particles of uniform size can be packed more efficiently, minimising voids in the column. Void spaces facilitate unwanted diffusion of analyte species, leading to broader peaks and reduced efficiency. Additionally, the absence of fines (smaller particulates that migrate towards the bottom of the column and block the end frit) resulted in lower backpressures and extended column life.
Superficially porous particle (SPP, or “core-shell”) columns, which were “reintroduced” in 2006 with AMT’s HALO® range, are well established for delivering high-efficiency separations at low pressures. This is thanks to the solid core of the particles, which can be manufactured with much tighter dimensional specifications. A reported downside of SPP particles is that they exhibit somewhat lower loadability than their fully porous counterparts, due to the shallower porous layer (Broeckhoven, 2013). Therefore, Cadenza and Unison columns can compete favourably, not only with sub-2μm columns but also with core-shell columns of similar selectivity, without compromising loadability and peak symmetry.
Stationary Phase Chemistry
The surface chemistry of a column, that is the stationary phase and surface end-capping is at least as important as the quality of the silica support. Several quality attributes are critical: chemical properties of the ligands, organosilane bonding type, bonding density (%C or surface coverage), end-capping chemistry, etc.
Many Imtakt stationary phases, beginning with the earliest Cadenza CD-C18 columns, have been designed with bidentate or tridentate ligands, rather than the more traditional mono-dentate:

Figure 1: Different types of silica surface modifications. 1: Bridged ‘polymeric’ substrate. 2: Low-energy silanol pair. 3: Metal-activated acidic site. 4: Monofunctional (monodentate) bonded ligand. 5: Lone acidic silanol. 6: Trimethylsilyl end-capping. 7: Difunctional (bidentate) ligand.
With two or more anchoring points (see figure 1, graphic 7 versus graphic 4), these ligands are intrinsically more stable to acidic hydrolysis, extending the useful pH range of the column.
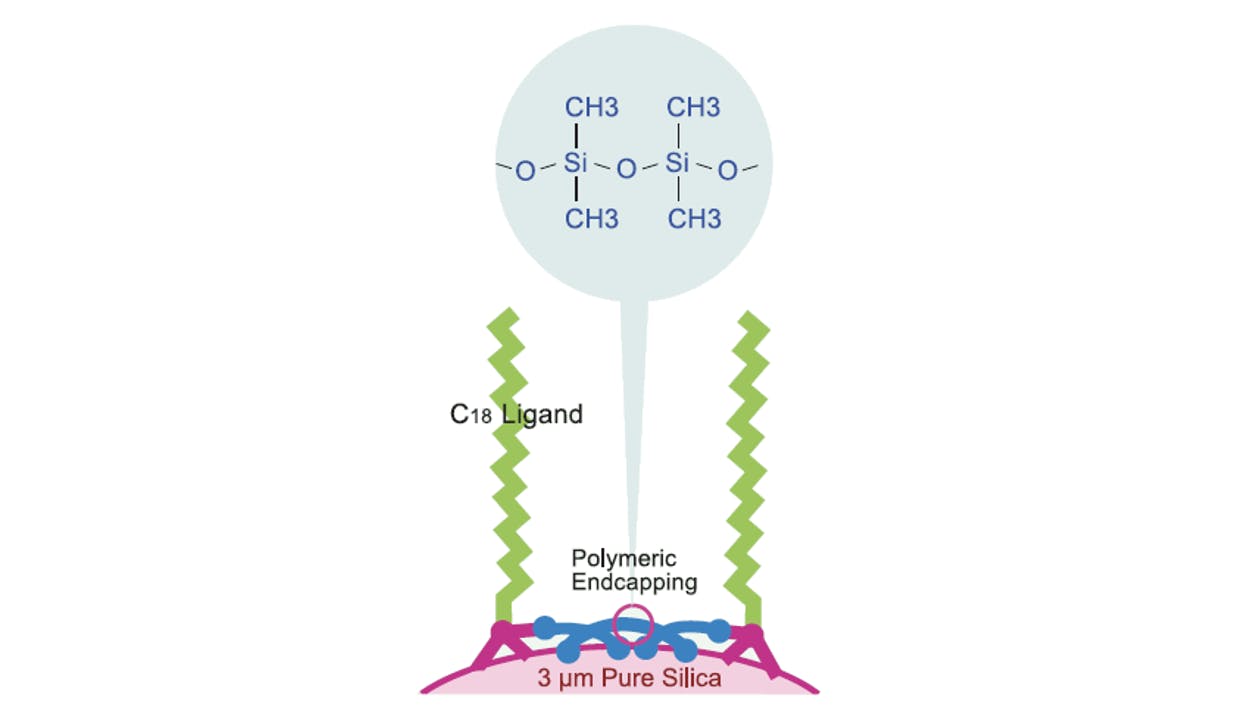
Figure 2: Polymeric endcapping used in Cadenza, Unison, and other Imtakt stationary phases. Adapted from Imtakt USA.
In traditional endcapping technologies, small functional groups like trimethyl silane (TMS, see schematic 6 in Figure 2) are applied to the silica surface after the main stationary phase ligand has been bonded. These small molecules diffuse easily into the porous silica surface, neutralising silanol groups that the main ligand, due to steric hindrance, would not be able to react with. So-called “lone” silanol groups (schematic 5), not stabilised through hydrogen bonding by neighbouring silanol moieties (schematic 2), are especially critical. These groups have a strong tendency to react as Bronsted acids and cause peak distortions, especially severe when analysing basic species.
Polymeric endcapping, unlike TMS, covers the silica surface with a network of organosilane chains. These short “polymers” accomplish two functions: on the one hand neutralise unreacted silanols, on the other, they provide some protection of the silica surface. This is important when operating at high pH ranges since silica can hydrolyse and dissolve in alkaline conditions. Polymeric endcapping combined with the difunctional ligands described above, effectively extends the range of an HPLC column from pH 1 to 12.
Polymeric endcapping proceeds through a series of steps. First, a number of “seed” organosilane monomers are bonded to the silica. In subsequent steps, the polymer network grows by condensation of additional monomers. Figure 5 suggests that the C18 on Cadenza and other Imtakt stationary phases would be involved in the polymerisation, thus the endcapping would further stabilise the ligands against extreme pH conditions.
The polymeric endcapping developed for Cadenza CD-C18 proved very successful and was later used in the other Imtakt families. The Cadenza range itself grew over the years to include a 300Å pore size variant (WP-C18), a polar-endcapped version (CX-C18), a low-density polymeric endcapping version, with exposed silanol residues for alternative selectivity (CL-C18)…
Cadenza HS-C18
One of the most innovative columns in the family was launched in 2006: Cadenza HS-C18, designed for the analysis of complex biological samples. HS-C18 is a 3μm silica particle with a proprietary hydrophobic/hydrophilic ‘hybrid’ stationary phase which combines two high-surface coverage, complementary ligands (C18 and PEG-like), with controlled-density polymeric endcapping:

Figure 3: Schematic diagram of the stationary phase of Cadenza HS-C18, showing hydrophobic and hydrophilic moieties. Adapted from Imtakt USA.
Due to the high ligand density, molecular species larger than approximately 30kDa are sterically excluded from the stationary phase, while small molecules of a wide range of polarities are efficiently retained.
With this unique chemistry, Cadenza HS-C18 was the world’s first column specifically engineered for direct injection of blood plasma. A very topical application demonstrates the size exclusion and low-molecular weight selectivity combination exhibited by this column:

Figure 4: Direct injection analysis of PFAS in bovine serum. Analytical conditions as indicated. Adapted from Imtakt USA.
PFAS (Perfluoroalkyl and Polyfluoroalkyl Substances) are a complex group of synthetic compounds that are widely used to manufacture everyday products, from cosmetics to food packaging, waterproof coatings, and stain-resistant fabrics. The widespread occurrence of PFAS, their as-yet unknown environmental persistence (they are known as “forever chemicals”), and their bioaccumulation potential has prompted the National Institute of Environmental Health Sciences (NIEHS) and other health agencies to fund extensive research on PFAS (Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS), n.d.).
Figure 4 shows an analysis of PFAS in bovine serum, injected onto Cadenza HS-C18 without previous sample preparation. The large band at the beginning of the chromatogram is produced by the protein fraction of the sample, which is sterically excluded and elutes in the column void. The small-molecule fraction, free from interference, shows a highly efficient separation with great peak shape.
Scherzo
A particularly interesting range in the Imtakt catalogue is the Scherzo family:

Figure 5: Diagram of the three Scherzo C18 stationary phases. Adapted from Imtakt.
Scherzo particles are derivatised with reversed phase, anion exchange, cation exchange, and normal phase functionalities, resulting in a mixed-mode zwitterionic-like stationary phase. They are not unique in merging multiple retention modes: similar columns include Amaze TR from Helix Chromatography (Amaze HPLC Columns), Obelisc R from SIELC Technologies (Obelisc HPLC Columns), or the Acclaim Trinity P1 from Dionex (Acclaim HPLC columns).
However, the chemistries and construction of all these columns are very different. Amaze TR and Obelisc R combine all three functional groups onto a single ligand, bonded to fully porous silica in a single step. Acclaim Trinity P1 is based on a so-called nano polymer-silica hybrid (NSH) geometry, which consists of 3μm porous silica particles, functionalised with a reversed phase – anion exchange mixed-mode stationary phase, and further coated with electrostatically attached sulfonated PS-DVB nanoparticles. While manufacturing these particles must be extremely challenging, Dionex claim that the spatial separation between positively and negatively charged groups ensures that both ion exchange mechanisms operate simultaneously and can be controlled independently.
Imtakt approached the problem of separating anion and cation exchange groups in a simpler way: the columns are packed with a 1:1 mixture of particles with different chemistries. Half of them are derivatised with reversed phase and anion exchange groups, the other half with reversed phase and cation exchange ligands. Separating chemistries in this way means that functionalisation is simpler and the final product is more robust and reproducible, provided that the two types of particles are packed homogeneously. Imtakt have published several application notes demonstrating excellent batch to batch reproducibility of all three Scherzo columns:

Figure 6: Batch to batch reproducibility of Scherzo SM-C18. Analytical conditions as indicated. Adapted from Imtakt USA.
Preparing a mixed-mode column from separate individual ligand moieties has an added advantage: by varying the chemistry of the anion and cation exchange functional groups, and the density of each group on the silica surface, Imtakt were able to develop three subtly different (see Figure 5) but complementary columns:
- The Scherzo SS-C18 contains a C18 chain supplemented with a high density of strong anion exchange (SAX) and strong cation exchange (SCX) groups. The strong ionic character of this phase enhances the retention of weakly ionised compounds, as well as zwitterions.
- The Scherzo SM-C18 has a moderate surface coverage of weak anion exchange (WAX) and weak cation exchange (WCX) functional groups, resulting in a lower ionic character that can be further modulated by varying mobile phase pH. The SM-C18 is effective at separating acidic and basic compounds in neutral pH.
- The Scherzo SW-C18 has the lowest density of SAX and SCX moieties and the weakest ionic-to-RP ratio. It is an efficient column for the separation of strongly ionised and basic species under mild conditions, especially suitable for LC-MS applications.
Additionally, operating the Scherzo columns with mobile phases above 50% organic reveals an additional “NP” retention mechanism. On the one hand, the highly organic eluent reduces the retention of hydrophobic compounds by RP. On the other, it deactivates ion exchange interactions due to the low dielectric constant. However, these conditions promote the adsorption of water on and around the ion exchange functional groups, creating a HILIC-like system on the silica surface that can aptly retain very polar solutes.
Balancing these seemingly conflicting retention mechanisms may seem complex, but it can lead to very sophisticated selectivity when done correctly. Beyond the proportion of aqueous-organic mobile phase solvents, pH is a crucial parameter that must be considered, and controlled, carefully:

Figure 7: Retention of a neutral compound, a sulfonic acid and a quaternary amine on Scherzo columns as a function of eluent pH. Adapted from Imtakt.
Figure 7 illustrates the response of all three Scherzo columns to changes in eluent pH. The retention of benzamide, a neutral compound, is unaffected by pH. The retention of benzenesulfonic (a strong acid) and phenyltrimethylammonium (a strong base) is, however, dramatically affected.
On Scherzo SS-C18 (the “strong” ion exchange phase) both acidic and basic compounds are strongly retained at low pH. As we approach neutral pH, retention drops due to a decrease in overall ionic strength. Scherzo SW-C18 (the “weak” ion exchanger) exhibits a similar behaviour, albeit less pronounced due to the lower surface concentration of SAX and CSX groups.
Scherzo SM-C18, however, responds differently to acidic and basic analytes. Low pH conditions neutralise the WCX groups (carboxylic acids), resulting in a positively charged stationary phase. A positive surface charge enhances the retention of acidic compounds, but excludes basic species. As eluent pH increases, a growing population of WCX groups become ionised, and a growing proportion of WAX groups (secondary or tertiary amines) become neutralised: surface charge shifts from positive to neutral, and eventually negative. The net effect is a reduction in retention of acidic species, but an increase in retention for basic compounds.
Buffer and salt concentration (i.e. ionic strength) are also important parameters to consider. The figure below illustrates the general behaviour of all three columns as eluent salt concentration is modified:

Figure 8: Retention of a neutral compound, a sulfonic acid and a quaternary amine on Scherzo columns as a function of eluent salt concentration. Adapted from Imtakt.
As expected, retention of benzamide is unaffected. For both strong acids and strong bases, we see retention decrease with increasing salt concentration. This trend can be explained by the change in electrokinetic (or zeta) potential on the charged surface of the particles. At low salt concentrations, with a sparse cloud of counterions surrounding the surface, the zeta potential extends a long distance from the particles, promoting the electrostatic attraction of both anions and cations. As the concentration of salt increases, surface charges become shielded by counterions, reducing the extent of the zeta potential (alternatively, competing with the analytes) and reducing retention times. The different extent of these effects on SS-C18, SM-C18 and SW-C18 reflects the differences in surface charge density between the three phases.
| Type of solute | Elution strategies for Scherzo C18 columns |
| Neutral, hydrophobic | Optimise organic solvent composition, as in conventional RP. Peak shapes can be improved with the addition of 0.1% acetic acid |
| Ionisable, hydrophobic | Optimise organic solvent and ionic strength. Add 20 – 100mM salt. Optimise pH for the separation of acids, bases, or both simultaneously. pH gradients are possible. |
| Weak ionic, polar | SS-C18 first choice. Increase ionic strength for compounds with multiple ionisable groups. Neutral pH for monofunctional carboxylic acids. |
| Strong ionic, polar | SW-C18 (weak retention, higher throughput) useful alternative to SS-C18 (too strong retention) and SM-C18. Optimise organic composition and ionic strength. Organic and salt gradients (simultaneous or sequential) are possible. |
Imtakt have compiled an extensive library of examples that demonstrate the complex separations possible on Scherzo columns, very often comparing all three columns under identical conditions. These examples provide valuable insights into the operating mechanisms of the three phases:
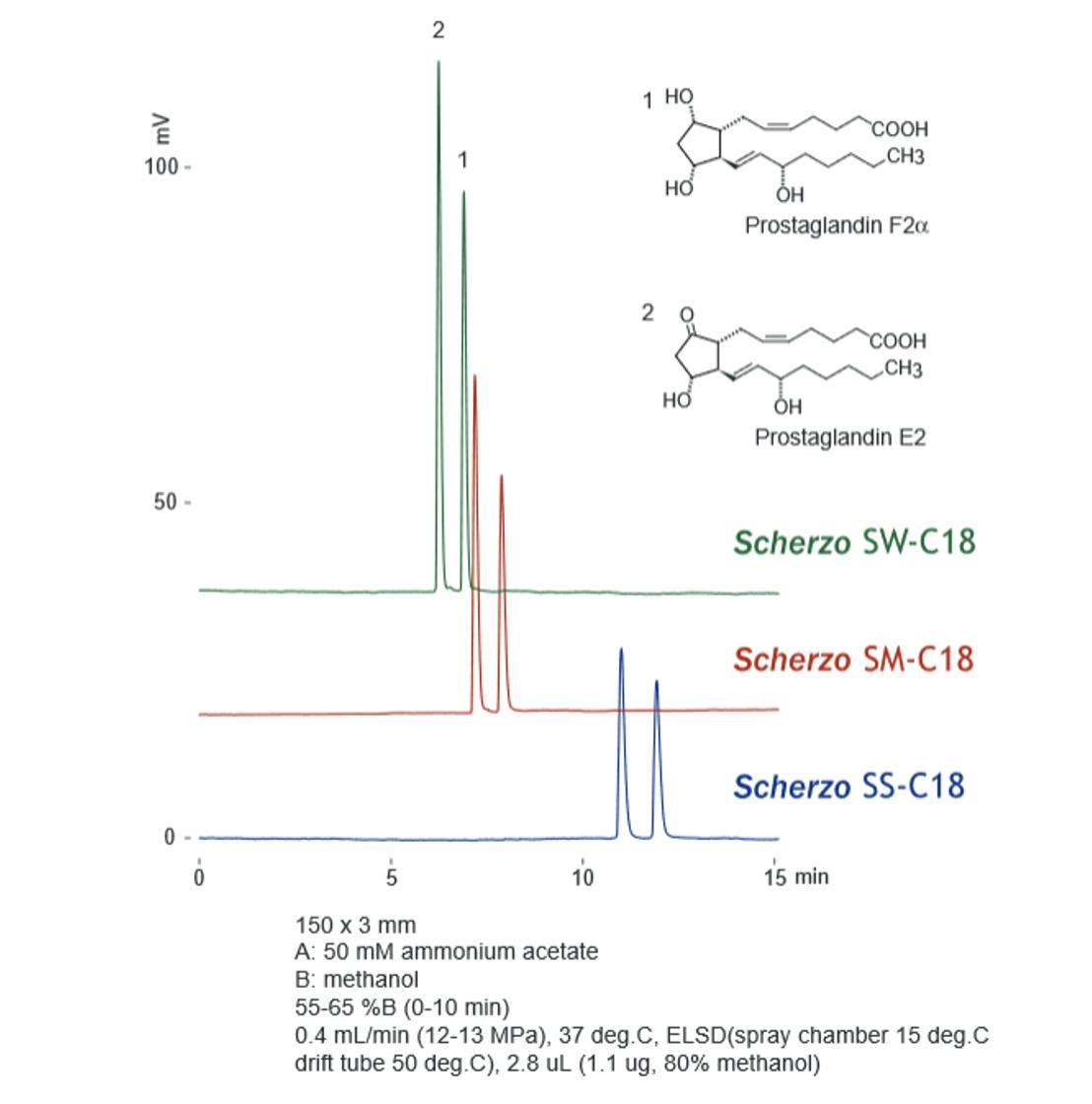
Figure 9: Analysis of prostaglandins on Scherzo SW-C18, SM-C18, and SS-C18. Adapted from Imtakt.

Figure 10: Analysis of serotonin-related compounds on Scherzo SW-C18, SM-C18 and SS-C18 (3x150mm). Analytical conditions as indicated. Adapted from Imtakt.
Other application notes demonstrate the unique capabilities of a particular Scherzo column applied to solve a specific, and often complex, separation:

Figure 11: Oligonucleotides on Scherzo SW-C18 3x150mm. Analytical conditions as indicated. Adapted from Imtakt.

Figure 12: Simultaneous separation of 15 fat- and water-soluble vitamins on Scherzo SM-C18 2x150mm. Analytical conditions as indicated. Adapted from Imtakt.
There is a lot more to learn about Cadenza, Unison, Scherzo, Intrada and Dacapo. Take a look around our new e-commerce site or browse imtaktusa.com for more application notes and product information.
As ever, we are here to help. Do not hesitate to contact us for additional information and sales support.
References
Broeckhoven, K. e. (2013). Kinetic performance comparison of fully and superficially porous particles with sizes ranging between 2.7um and 5um: Intrinsic evaluation and application to a pharmaceutical test compound. Journal of Pharmaceutical Analysis, 313-323.
Perfluoroalkyl and Polyfluoroalkyl Substances (PFAS). (n.d.). Retrieved from National Institute of Environmental Health Sciences: https://www.niehs.nih.gov/health/topics/agents/pfc/index.cfm