20 May 2019
HPLC Troubleshooting Filtration
Even when you have carefully chosen a suitable method and equipment for HPLC, problems can still occur, and troubleshooting becomes an unfortunate use of valuable time. When troubleshooting, sample preparation is often a key consideration. So, what are some of the possibilities you might consider regarding your sample preparation filtration techniques?
Why is filtration recommended?
Although some high throughput approaches may not allow for filtration, filtering your sample as a minimum preparation step is highly recommended. Unfiltered particulates in samples can often clog instrument interfaces and HPLC columns, causing increased backpressure or blocking the device altogether. Filtering samples is a simple, cost-effective way of removing particulate material from your sample, thereby increasing instrument and column longevity, right-first-time rate of analysis, and potentially providing better chromatographic results.
Filtration is usually a way to physically remove particulate species out of solution. Generally, there is no chemical separation exerted by the filtration and, if sample clean-up is required, you may need to consider other, more selective, sample preparation methods (i.e. solid phase extraction) (Table 1). One notable exception is ultrafiltration, where pressure or concentration gradients are used to force separation through a semi-permeable membrane.
Fundamentally, filtration is a simple and actionable method to improve your analytical outcomes. So how do you go about selecting the correct syringe filter.
| Filtration Media | Products | Use |
| Filter paper | Cellulose | Removal of large particles (> 40 µm) |
| Membrane filters | Nylon, PTFE, polypropylene, polyester, PES etc. | Removal of small particulates (< 10 µm, generally down to 0.2 µm) |
| Functionalized membranes | Ion exchange, affinity membranes | Removes particulates and matrix interferences |
| SPE cartridges | Silica and polymer based | |
| SPE discs | PTFE and fiber glass based |
Table 1: Filtration media
Selecting a Syringe Filter
The type of filtration product you choose needs to be based on the desired outcome of the analytical technique you intend to use. Filter paper can remove particulates which are greater than 40 µm in size and provides an initial step in filtering particularly dirty sample when followed by a further filtration step. However, since a sample that has been through filter paper is going to contain 40 µm particulates, it will be ineffective in filtering a sample before injecting on an HPLC column. As an example, a typical HPLC column may have particle sizes of 5 µm and, therefore, a frit of 2 µm, which will become blocked very quickly. Table 1 gives an overview of some of the techniques to consider when selecting a suitable filtration method.
Syringe filters are the most common and cost-effective method of filtering samples for HPLC. We have put together an infographic outlining the importance of syringe filters. Check it out below.
The selection of syringe filter material and characteristics may not seem like the most important decision in the grand scheme of method development; however, if careful consideration is not given to the type of membrane, filter size (diameter), and porosity, then problems can arise.
Solvent Compatibility
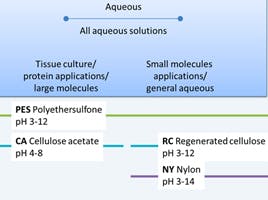
One of the first parameters to consider would be the diluent’s compatibility with the membrane material (Figure 1). Not choosing the correct filter membrane is a common problem that creates issues in HPLC; such as the introduction of extraneous extractables and reducing the detection of analytes. Furthermore, solvents that are incompatible can cause swelling of the filter polymeric material which will affect performance and filtration efficiency.
Membranes such as PFTE are only compatible with organic solvents, whereas some membranes — like polyethersulfone (PES) — can tolerate all aqueous diluents and some organic solvents. In order to avoid damaging the syringe filter and impacting the integrity of your results, the exact chemical compatibility of your syringe filter should be checked with the manufacturer/distributor — most provide detailed compatibility tables that consider organic solvents, bases, aqueous components (i.e. hydrogen peroxide), and solution pH in order to avoid damaging the syringe filter due to inappropriate use.
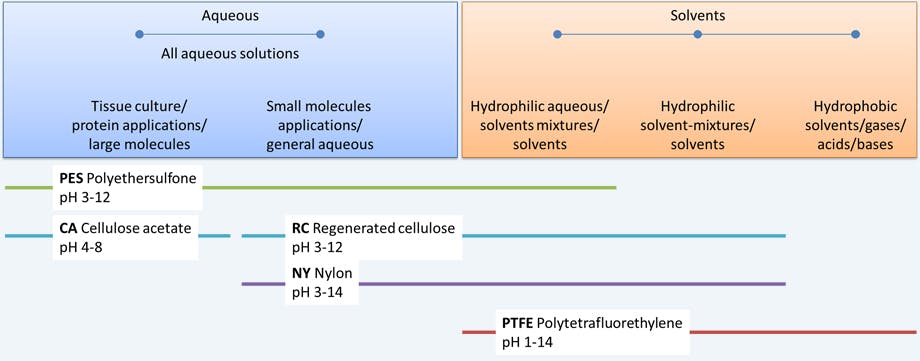
Figure 1 Consideration of sample composition when selecting a syringe filter.
Another factor to consider is the housing that holds the membrane. Most commonly, the housing is made of polypropylene (PP) or methacrylate butadiene styrene (MBS) which will exhibit different chemical resistance and could be damaged by your diluent or suffer from leaching of material from the filter (which could contaminate your sample).
Use our quick, interactive syringe filter selection guide below to find out what filter is best suited to your analysis. In a few clicks you can submit your recommendations and we will send you a free, personalised sample pack.
You can find our full range of syringe filters here »
Non-specific binding
Once it’s been ascertained that the diluent is compatible with your syringe filter, turn attention to the type of analytes present in the sample. Non-specific binding of the analyte to your filter could result in inaccurate quantification, especially if you are dealing with low concentrations of sample. For example, glass fibre filters tend to bind strongly to proteins, peptides, and oligonucleotides. It is also very important to check for non-specific binding of an analyte. Screening 2 or 3 different type of syringe filters to ensure the best selection of filter material is recommended.
Filter clogging
If a sample is particularly viscous or laden with particulate, then a single membrane filter membrane gets clogged before the sample reaches and passes through the syringe filter. When working with these types of samples it is advantageous to use a syringe filter with a prefilter. The prefilter will trap larger particulates and protect the final membrane from fouling thus allowing higher volumes of sample to be filtered before the filter clogs. The caveat being that the prefilters in multi-layered syringe filters are usually made of glass fibre which as discussed earlier, can lead to a greater amount of analyte binding, especially with proteins, peptides, and oligonucleotides. Glass fibre can also lead to higher levels of extractables leaching into your sample. If these are issues, it may be wise to stick to single layer syringes and prefilter using an increased porosity to try to reduce blockages when using the final desired porosity level filter.
Choosing the right size filter
The ideal filter diameter (2-50 mm) balances performance with the risk of extractables and analyte binding. This will depend on the sample volume (Table 2). Larger filters will provide faster filtration with lower pressure (minimising the chance of bursting the membrane); however, the trade-off is that they have higher hold-up volumes, which could trap sample, resulting in greater sample loss and poor quantitation’s. The use of large filters also equates to greater analyte loss due to nonspecific binding and potentially higher levels of extractable impurities.
| Sample Volume (mL) | Filter Diameter (mm) | Hold-up volume (µL) | Filtration Area (cm2) |
| < 1 | 4 | 10 | 0.1 |
| 1-10 | 13 | 25 | 0.65 |
| 10-150 | 25 | 100 | 3.6 |
| 10-150 | 33 | 80 | 4.5 |
Table 2: Syringe filter diameters (Note diameters may vary slightly between manufacturers).
Extractables
Extractables are undesired artefacts that sample fluid from the filter device may contribute towards. These components are extracted from the filter by the sample solvent and may cause erroneous signals within your chromatogram or mass spectrum, which could affect quantitative results. However, a simple way to reduce these impurities is rinsing the filter with solvent prior to carrying out filtration of the sample (Figure 2). Evaluating the amount of extractable generated in a method, whenever possible, is recommended.
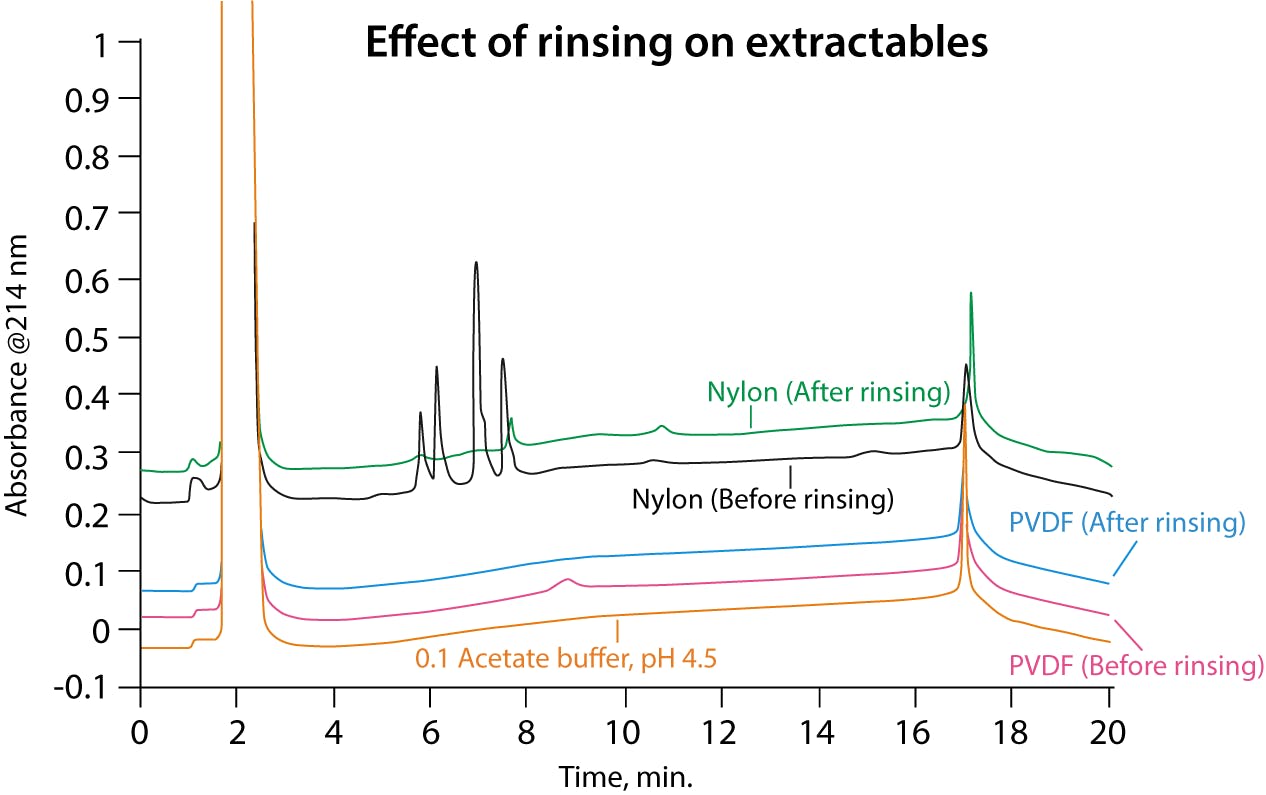
Figure 2 Effect of rinsing on extractables.
Evaluating Filtration Efficiency
Equation 1:
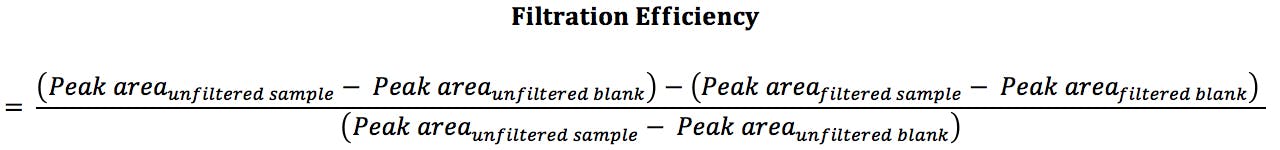
Finally, in order to evaluate your filtration method when troubleshooting, or to help when testing between different syringe filter samples, it is necessary to determine filtration efficiency. This is done by comparing the peak area of your analyte of interest in an unfiltered sample compared with the filtered sample (Equation 1). The sample used should be the specific sample under investigation. If this is unavailable, or you have several samples, a representative sample should be chosen. The optimum filter will be the one with highest efficiency (i.e. maximum recovery of your analyte of interest) whilst ensuring contaminants are not introduced into your sample by the filtration process.
References
https://www.crawfordscientific.com/technical/syringe-filter-selection-guide
https://www.chromacademy.com/chromatography-Troubleshooting-Filtration.html