31 Jan 2022
MythBusters: Solvent safety is only a health and safety issue
Health and safety improvements are always on our minds. But we come across a lot of conjecture on this topic, in the context of solvent storage and caps, when we speak with people working in labs.

In this MythBuster we are going to review some critical aspects of lab safety around solvents and (hopefully) change some people’s minds on how they operate, busting 3 common myths wide open:
-
- If I change my solvents regularly, I don’t need solvent safety caps
- Waste safety caps are unnecessary if my lab is sufficiently ventilated
- Aqueous phase products don’t pose an H&S risk
Myth 1: I change my solvents regularly, I don’t need solvent safety caps
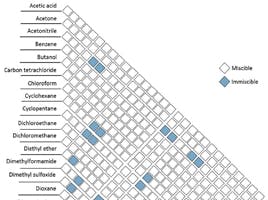
To be fair, the main reason to use solvent safety caps isn’t really for health and safety, the issues mostly come from the waste bottles (we will come back to that). But this will enhance your solvent integrity by preventing evaporation of some volatile components (i.e., 1% NH3 in Methanol) and also prevent contamination of the mobile phase from ambient molecules. A recent analysis performed for S.C.A.T. demonstrated that up to 2% of the volume in the mobile phase can change over the course of only 8 days if caps are not being used properly (i.e. if people are using parafilm or tin foil). There can also be a strong modification of the proportions within your mobile phases, which will lead to poor data generation and elution time-shifting.

Figure 1: Determination of the density and change of volume of methanol/water mixture (Source SCAT)
Another test demonstrated that using solvent safety caps is the equivalent of having your solvent stored in a completely capped bottle (at least for 31 days). Ultimately, it’s a very simple, cost-effective way to improve data reproducibility and also save money by keeping your mobile phase longer.

Figure 2: Weight loss of solvent bottles over a period of 31 days (Source SCAT)
Myth 2: Waste safety caps are unnecessary if my lab is sufficiently ventilated
Renewing the air in the lab is vital for good operations, both for the generation of data and to preserve the health and safety of the scientist spending hours in the lab.
Most people would believe the ventilation in the lab is sufficient to prevent the accumulation of toxic solvents that pose a risk to staff, and this is likely to be true if you ignore the fact that people often develop a headache when standing close to a piece of equipment for multiple hours. The worst part of this is that people can get used to a specific smell. We have visited numerous labs where the latent smell of organic solvent is stark, but all the scientists from the lab couldn’t smell a thing!
Having a waste canister capped with an efficient filter will considerably reduce the organic and acid smell present in your lab while maintaining a higher level of safety.
Go from this:

To this:

Myth 3: Aqueous phase products don’t pose an H&S risk
Did you know that one of the main causes of accidents in the analytical lab come from solvent spillage and overflowing HPLC solvent bottles? The reason is simple, in a set-up where people are sharing equipment, it is everyone’s (and therefore no one’s) responsibility to take care of the waste solvent bottle. How many times have you found out that the waste bottle overflowed? When you find the waste canister floating in the tray in a sea of waste solvent. And that’s if you have a solvent tray, which is generally implemented the first time people had an overflown waste canister.
This poses 2 risks: possible toxic solvent emanations but also the risk of slipping and falling. Most of the time this will be considered as unidentified waste due to the uncertainty of what is used in the mobile phase over time. This will possibly push you to go for the emergency shower (something nobody really wants in the lab!)
There is an increasing demand to better control the flow of waste. And we have the perfect solution.
The Symline range helps scientists to design their flows of waste solvent around their HPLC system and optimize the collection into a central canister. This reduces the risk of overflowing with a long-term objective of reducing costs. If you want to know more about this, please contact us.
And that concludes another round of MythBusters. We hope that we have changes some minds and helped you to be safer with solvents.