02 Apr 2019
Superficially porous particles
Since the revolutionary and well-named Halo particle was introduced in 2007 by Advanced Material Technologies, most HPLC column manufacturers have created similar products. A few weeks ago, our very own Tony Taylor reminded us in his blog post (Have we forgotten the advantages of core-shell particles?) how sub-3μm superficially porous particles (SPPs) have become popular for method development and optimisation on regular HPLC systems [1]. Their efficiencies approach that of sub-2μm fully porous particles (FPPs) and the lower pressure spares labs the additional need and cost of purchasing UHPLC instruments.
The technology is not confined to small-molecule, reverse-phase applications anymore. Most notably, it has been successfully applied to large-molecule analysis, as well as entering the sub-2μm UHPLC and HILIC arenas. The latest applications include chiral and size exclusion chromatography.
Superficially porous particles and loading capacity
The most common criticism we hear from labs is the supposed limited quantity of material which can be injected on to SPPs. Columns packed with SPPs are regularly said to be prone to saturation. As a result, I have been advised not to use SPP columns with complex samples, such as plant extracts, as this would lead to abnormal peak shapes and poor resolution. However, I have also met analysts that are happy doing routine small-scale purifications of natural products and peptides on semi-preparative SPP columns.
Right or wrong? Should an analyst go with gut feeling or rational comparison with the FPP equivalents? Well, the loading capacity of a column can generally be estimated by its specific surface area and the amount of phase bonded to the silanols of the silica particle. As SPP cores are unavailable for analyte interaction with the stationary phase, it seems logical that their loading capacity is negatively impacted by the particle geometry. However, looking at the data, this statement tends to be less evident.
SPPs are predominantly porous
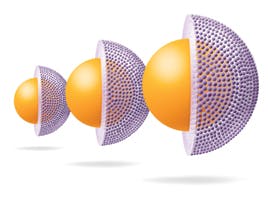
Advanced Material Technologies launched the 2.7μm Halo particle in 2007. They selected a particle size and core to shell ratio that would optimize the loading capacity, with 1.7μm core and 0.5μm porous shell sizes identified as the optimum for each. Roughly 75% of this particle is porous. Since this first attempt, the relative ratios of the porous volumes between the sub-3μm SPPs and the FPPs of identical diameters range from 0.61 to 0.76. The superficially porous spheres remain predominantly porous.
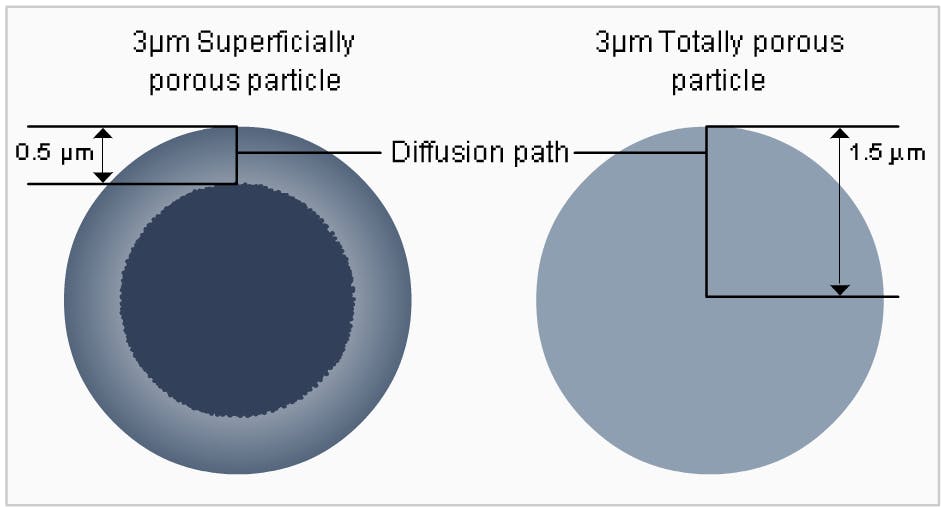
Fig1: Different particle geometries: superficially porous particles (SPPs) vs. fully porous particles (FPPs). The 0.5μm porous shells account for 75% of the volumne of 2.7μm SPPs.
SPPs allow for a better packing
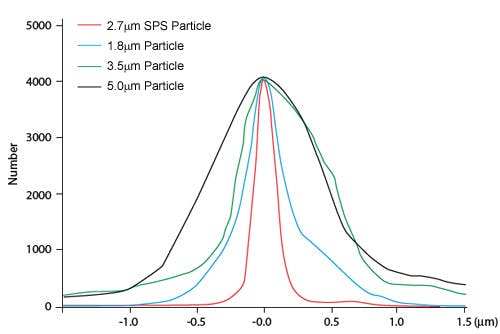
It is accepted that the narrower particle size distribution and the higher density of SPPs make key contributions to the superior efficiency of SPPs over their FPP equivalents. This results in a more homogenous and compact packing (more SPP phase in a given hardware).
Fig 2: Particle size distribution of different SPPs and FPPs.
The particle efficiency benefits from this packing and very low eddy dispersion known as the A-term of the famous Van Deemter equation, has been observed for sub-3μm SPPs. [2] to [5]
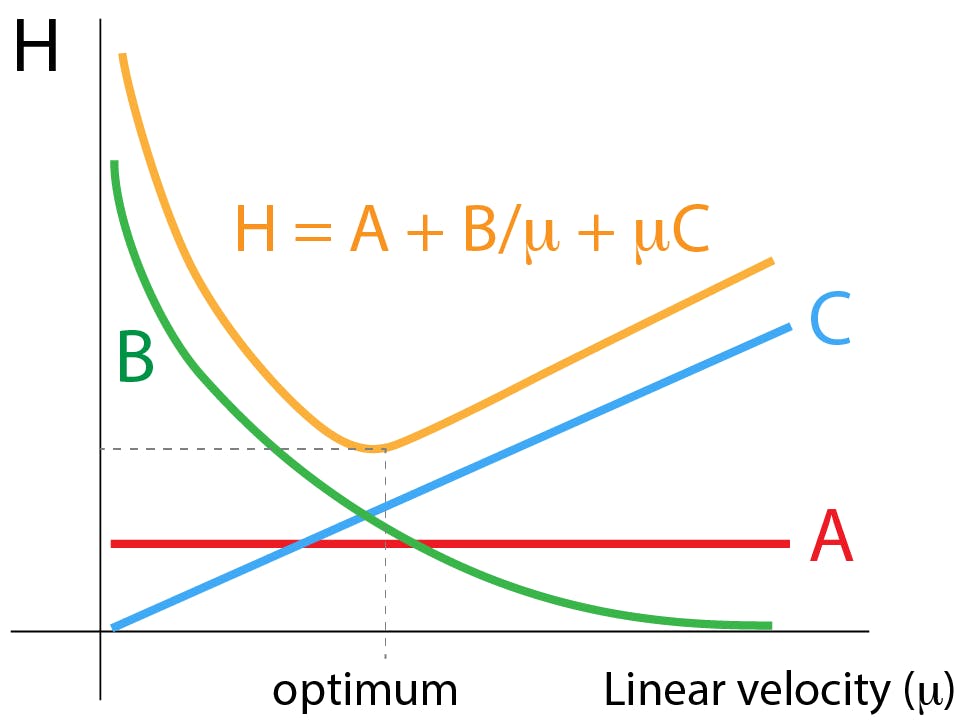
Fig 3: Van Deemter: contributions to peak broadening. A - Eddy dispersion; B - Longitudinal diffusion; C - Resistance to mass transfer.
Several studies show that SPP loading capacities do not differ much from that of FPPs
One accepted definition of column overloading is the 10% reduction in efficiency (or the 10% increase in peak width) when injecting increasing masses of an analyte. Several studies showed that the saturations of FPPs and SPPs occur roughly at the same point, suggesting that the dramatic impact on the loading capacity is not as bad as initially anticipated.
Fallas et al. showed back in 2012 that “the capacity of shell particles was not greatly reduced” and that buffer concentration was key in order to increase loading capacities of ionized analytes. Higher loading capacities were recorded for both acidic and basic probes at low pH, and the buffer strength increase from 5 to 100mM ammonium formate resulted in a 10-fold increase of loading capacities for SPPs and FPPs [6]. This was also verified by Ron Majors in his 2013 article “the top ten HPLC and UHPLC column myths” [7].
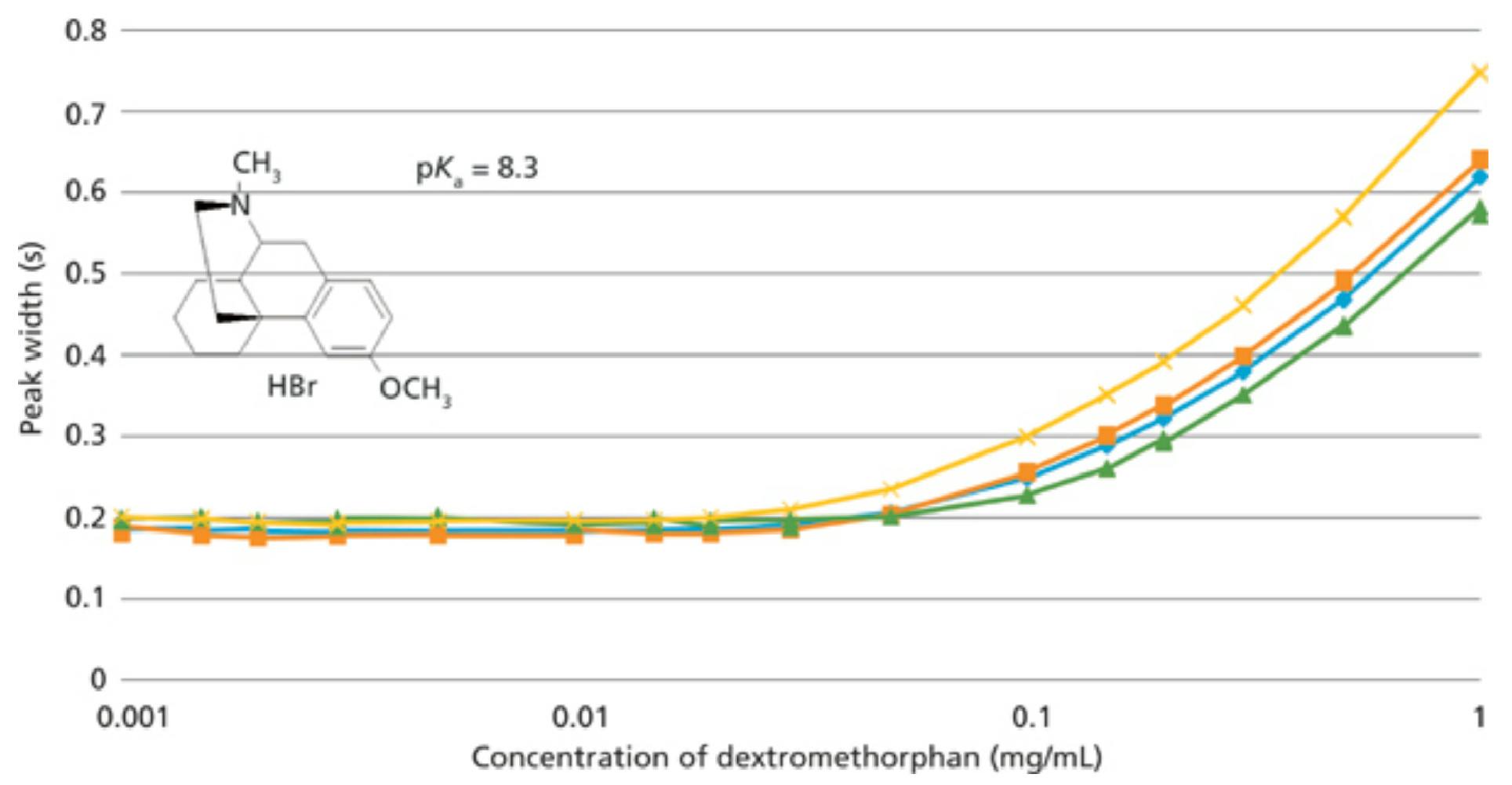
Fig 4: Sample loading of a basic compound (dextromethorphan) onto superficially porous and sub-2-µm totally porous columns. A 10% increase in peak width for the superficially porous particle columns occurs roughly at the same loading as the 1.8-µm totally porous particle column. Mobile phase: 80% 25 mM Na2HPO4 buffer (pH 3.0), 20% acetonitrile; detection: UV absorbance at 205 nm; temperature: 30 °C. Columns: blue diamonds: 100 mm × 3.0 mm, 2.7-µm dp Agilent Poroshell 120 EC-C18; orange squares: 100 mm × 3.0 mm, 2.7-µm dp Supelco Ascentis Express C18; green triangles: 100 mm × 4.6 mm, 2.6-µm dp Phenomenex Kinetex C18; yellow X’s: 100 mm × 3.0 mm, 1.8-µm dp Agilent Zorbax Eclipse Plus C18.
Ron Majors, 2013 in: "The top ten HPLC and UHPLC column myths"
Ruta et al. showed that “concerning (SPP) loading capacity, it remains comparable to that of fully porous sub-2μm particles”. More than the shell thickness, it was “better correlated to the pore size or the surface coverage” [8]. This suggests that analytes do not diffuse much through the center of FPPs and that the impact of the solid core of SPPs on loading capacity is therefore lessened.
Superior efficiencies and similar loading capacities: SPP columns for small-scale purifications?
Since the introduction of sub-3μm SPPs, manufacturers have launched larger (3.5 to 5μm) SPPs for different applications [9]. The particles dedicated to large biomolecules, such as peptides and proteins, have seen their pore size increased from 150 to 450A, with more recent advancements reaching 1000A.
The shell thickness has also been adapted, with thinner shells resulting in higher efficiencies for large analytes [10] to [12]. The loading capacities of such columns must be affected by these new core/shell ratios. But small-molecule columns are also available, and their geometries are very similar to the initial sub-3μm SPPs [9]. When only a small amount of material is needed for further assays, it is tempting for the analyst to scale-up from the analytical method developed on an SPP analytical column and purify the targeted molecule on the corresponding prep column.
Chiral chromatography is, with size exclusion chromatography [11],[13], the latest field where the superior efficiencies of SPPs have been demonstrated. Higher enantioselectivity of chiral SPPs has been observed and chiral separations can now be achieved in less than a minute [14] to [17]. Scaling up for purification purposes is quite common in chiral chromatography as chemists look at purifying their intermediates to carry on with their synthesis. There could well be a niche for both analytical and semi-prep SPP chiral columns. Based on these results, we can expect manufacturers to put more effort in to developing semi-preparative columns packed with SPPs for fast, small-scale purifications.
References
[1] Taylor, 2019, “Have we forgotten the advantages of core-shell particles?”
[2] Majors, 2010, “The increasing role of superficially porous particles in HPLC”
[3] Guillarme and Fekete, “Advantages and applications of revolutionary superficially porous particle columns in liquid chromatography”
[4] Hayes et al., 2014, “Core-shell particles: preparation, fundamentals and applications in high performance liquid chromatography”
[5] Fekete et al., 2014, “Superficially porous particles: perspectives, practices and trends”
[6] Fallas et al., 2012, “A comparison of overload behaviour for some sub 2 um totally porous and sub 3 um shell particle columns with ionised solutes”
[7] Majors, 2013, “The top ten HPLC and UHPLC column myths”
[8] Ruta et al., 2012, “Evaluation of columns packed with shell particles with compounds of pharmaceutical interest”
[9] Broeckhoven et al., 2013, “Kinetic performance comparison of fully and superficially porous particles with sizes ranging between 2.7 μm and 5 μm: Intrinsic evaluation and application to a pharmaceutical test compound”
[10] Wagner et al., 2012, “Superficially porous particles with wide pores for biomacromolecular separations”
[11] Wagner et al., 2017, “Superficially porous particles with 1000Å pores for large biomolecule high performance liquid chromatography and polymer size exclusion chromatography”
[12] Fekete et al., 2018, “Tips, tricks and troubleshooting for separations of biomolecules. Part 1: Contemporary reversed-phase protein separations”
[13] Schure and Moran, 2017, “Size exclusion chromatography with superficially porous particles”
[14] Patel et al., 2015, “Gone in seconds: Praxis, performance and peculiarities of ultrafast chiral liquid chromatography with superficially porous particles”
[15] Bezhitashvili et al., 2018, “Application of cellulose 3,5-dichlorophenylcarbamate covalently immobilized on superficially porous silica for the separation of enantiomers in high-performance liquid chromatography”
[17] Agilent Poroshell 120 Chiral Application compendium