Chromatography Method Development
We’ll help you develop the right chromatography method. When you are starting from scratch, developing a new method can be a daunting prospect, especially if you have a lack of expertise or experience in that particular area. But dont panic just yet.
Our technical team have extensive experience in method development, gained from years of working in busy commercial labs. Whether you are a seasoned developer or new to the science, we are here to give you all the advice and assistance you need.
We have several of the common chromatography and mass spec software packages, allowing you to email us raw data to support your enquiry.
It’s also possible to hire one of our team to work with you at your site, or remotely, to either develop a specific method or to assist you in setting up a method development approach in your lab.
The approaches to development between HPLC, GC and SPE are totally different.
HPLC Method Development
Ideally, all methods should initially be developed using additives that are compatible with mass spec. Even if you’re not using mass spec, volatile additives tend to give much less wear to HPLC equipment. It’s important to follow these steps:
- Verify analyte solubility and select a suitable solvent / sample prep for samples.
- Select a suitable method of detection. This may have to be in tandem with step 4.
- Choose a suitable mode (Reversed Phase, HILIC, Normal Phase, Mixed Mode) to obtain retention.
- Preliminary scouting gradient in chosen mode to get a measure of analyte retentivity.
- Screen selectivity factors i.e. variables that can only be determined experimentally. This is usually the column chemistry, pH and the type of organic modifier. Screening would be performed on representative samples containing interference, to evaluate which conditions give the best preliminary separation and peak shape.
- Further optimise the best conditions obtained in Step 5. This is usually column dimensions and mobile phase (gradient) conditions.
- Prevalidation. A small run of samples that provide a preliminary indication of the method performance – precision, recovery, linearity and specificity.
- Validation.
GC Method Development
- This approach is equally applicable to GC-MS. We follow these steps in method development for GC.
- Verify analyte solubility and select a suitable solvent / sample prep for samples.
- Select a suitable method of detection. This may have to be in tandem with step 3 / 4.
- Review existing applications to give indication of preliminary conditions.
- Using a standard temperature program, screen 2-3 columns using test injections of a representative sample containing all expected compounds and interference.
Normally this would be a low, intermediate and high polarity capillary phase. - Optimise the best conditions obtained in Step 4. This would normally be the temperature program and inlet conditions.
- Prevalidation. A small run of samples that provide a preliminary indication of the method performance – precision, recovery, linearity and specificity.
- Validation.
SPE Method Development
With some basic method development we can nearly always obtain a method that gives cleaner samples and better recoveries than generic conditions. Try our little exercise which illustrates the principles of developing a method for SPE
- Based on sample type decide if it is easier to retain target analyte(s) or retain interference.
- Choose a suitable cartridge based on the retention mode required for Step 1 – this could be reversed phase, ion exchange, mixed mode or normal phase.
- Perform initial screen using default conditions (available on request) for the cartridge type being evaluated. This often provides a workable method.
- If default conditions don’t provide a solution, a load / wash / elute experiment is performed. This is simple and quick experiment where increasing concentration of wash / elution strength are evaluated. Each section of eluent is collected and analysed to track the behaviour of the target compound. We are experienced in this approach and can provide all the advice you need to set up this on your particular application. The following interactive experiment demonstrates this approach.
Prevalidation. A small run of samples that provide a preliminary indication of the method performance – precision, recovery, linearity and specificity. - Validation.
We’ll help you develop the right chromatography method
When you are starting from scratch, developing a new method can be a daunting prospect, especially if you have a lack of expertise or experience in that particular area. But dont panic just yet.
Our technical team have extensive experience in method development, gained from years of working in busy commercial labs. Whether you are a seasoned developer or new to the science, we are here to give you all the advice and assistance you need.
We have several of the common chromatography and mass spec software packages, allowing you to email us raw data to support your enquiry.
It’s also possible to hire one of our team to work with you at your site, or remotely, to either develop a specific method or to assist you in setting up a method development approach in your lab.
The approaches to development between HPLC, GC and SPE are totally different.
HPLC Method Development
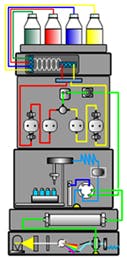
Ideally, all methods should initially be developed using additives that are compatible with mass spec. Even if you’re not using mass spec, volatile additives tend to give much less wear to HPLC equipment. It’s important to follow these steps:
- Verify analyte solubility and select a suitable solvent / sample prep for samples.
- Select a suitable method of detection. This may have to be in tandem with step 4.
- Choose a suitable mode (Reversed Phase, HILIC, Normal Phase, Mixed Mode) to obtain retention.
- Preliminary scouting gradient in chosen mode to get a measure of analyte retentivity.
- Screen selectivity factors i.e. variables that can only be determined experimentally. This is usually the column chemistry, pH and the type of organic modifier. Screening would be performed on representative samples containing interference, to evaluate which conditions give the best preliminary separation and peak shape.
- Further optimise the best conditions obtained in Step 5. This is usually column dimensions and mobile phase (gradient) conditions.
- Prevalidation. A small run of samples that provide a preliminary indication of the method performance – precision, recovery, linearity and specificity.
- Validation.
GC Method Development
This approach is equally applicable to GC-MS. We follow these steps in method development for GC.
- Verify analyte solubility and select a suitable solvent / sample prep for samples.
- Select a suitable method of detection. This may have to be in tandem with step 3 / 4.
- Review existing applications to give indication of preliminary conditions.
- Using a standard temperature program, screen 2-3 columns using test injections of a representative sample containing all expected compounds and interference.
Normally this would be a low, intermediate and high polarity capillary phase. - Optimise the best conditions obtained in Step 4. This would normally be the temperature program and inlet conditions.
- Prevalidation. A small run of samples that provide a preliminary indication of the method performance – precision, recovery, linearity and specificity.
- Validation.
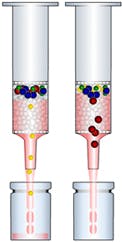 SPE Method Development
SPE Method Development
With some basic method development we can nearly always obtain a method that gives cleaner samples and better recoveries than generic conditions. Try our little exercise which illustrates the principles of developing a method for SPE
- Based on sample type decide if it is easier to retain target analyte(s) or retain interference.
- Choose a suitable cartridge based on the retention mode required for Step 1 – this could be reversed phase, ion exchange, mixed mode or normal phase.
- Perform initial screen using default conditions (available on request) for the cartridge type being evaluated. This often provides a workable method.
- If default conditions don’t provide a solution, a load / wash / elute experiment is performed. This is simple and quick experiment where increasing concentration of wash / elution strength are evaluated. Each section of eluent is collected and analysed to track the behaviour of the target compound. We are experienced in this approach and can provide all the advice you need to set up this on your particular application. The following interactive experiment demonstrates this approach.
- Prevalidation. A small run of samples that provide a preliminary indication of the method performance – precision, recovery, linearity and specificity.
- Validation.