20 Sep 2017
HPLC Column - Pore Sizes and Particle Diameters
The physical characteristics of silica based HPLC columns can affect the performance of the separation almost as much as the bonded phase. These are some of the lesser known, often forgotten facts relating to silica particles used for chromatography.
Silica particle manufacture
Think of it this way, there are only about a dozen, or so, companies who manufacture silica for HPLC packing materials. Many others bond stationary phase to silica from these manufacturers. Then, hundreds of column vendors ultimately put these to market — the PQRI column database contains over 600 different columns from a host of different companies.
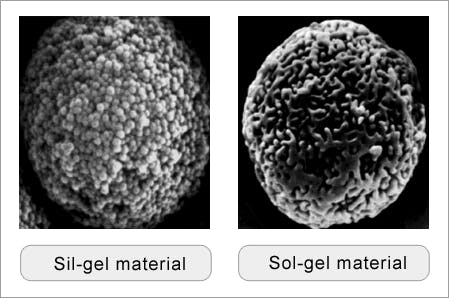
Silica particles used in chromatography are based on synthetic silica polymers. They are typically formed from tetraethoxysilanes, which are partially hydrolysed to polyethoxysiloxanes to form a viscous liquid which can emulsify in an ethanol water mixture. Stirring this creates spherical particles that are converted to silica hydrogels through catalytically induced hydrolytic condensation (the ‘Unger’ method) — causing extensive crosslinking via the surface silanol species. The hydrogel spheres are then heated (dried) to produce a xerogel. The pH, temperature, catalysts and solvents, as well as the silica sol concentration, all act to control the particle and pore size of the highly porous silica xerogel (sometimes called sol-gel) materials.
There’s an alternative process which uses silica microparticles. These are aggregated in solution using a urea/formaldehyde reagent to produce particles consisting of agglomerated microspheres — the so called ‘sil-gel’ materials. The concentration and diameter of these microparticles, as well as the reaction conditions, control the size of particles and pores of the resulting sil-gel particle.
Particle Size
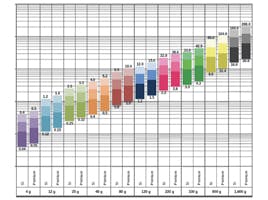
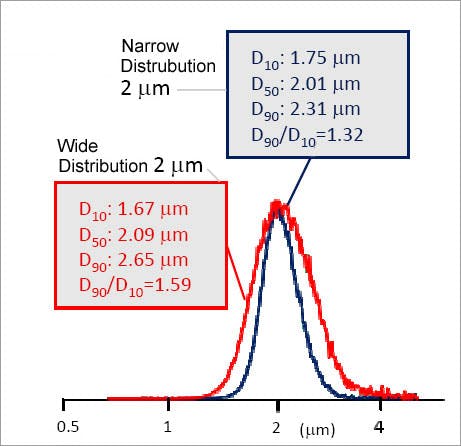
The particle size of the silica spheres controls the efficiency of the silica material, referencing the average diameter of the silica particles used for column packing. It should be noted that the particle size quoted is the average value, which comes from a range of particle sizes. The smaller the particle size distribution, the more efficient and robust the packing material. Manufacturers often quote a D10/90 ratio – which is the ratio of particle sizes at the 10th and 90th percentiles of the normally distributed range of particles. This indicates the breadth of the particle size distribution with a value of 1.2 or lower considered very good.
Smaller particles produce more highly efficient separations, or — perhaps more usefully — equivalent separations using smaller packed beds at high eluent flow rates.
Both columns back pressure is inversely proportional to the particle size, according to the following relationships;
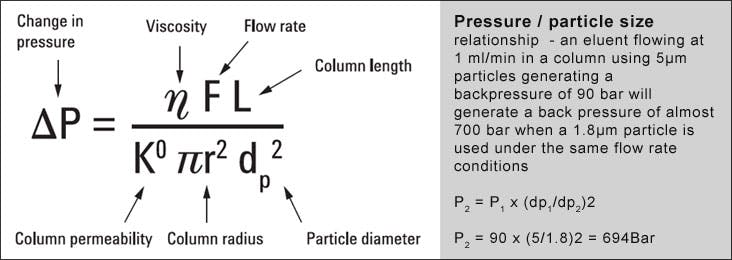
Chromatographic efficiency is inversely proportional to particle size according to the following general relationship:
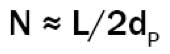
Where L is the column length and dP the particle diameter.
Therefore, changing from a 5µm to 3µm particle and keeping all other factors constant will produce an increase of 20 -25% in efficiency and subsequently increase chromatographic resolution by around a 9 - 12%.
Small particles (<2µm) are typically used for high-resolution separations using longer columns, or high speed separations using shorter columns. They often require the use of specialist HPLC systems capable of dealing with high back pressures.
Intermediate particle sizes (2 – µmm) are typically used for more complex separations of similar components where ultra-high high speed is not a primary factor.
5 – 10µm particles are used for routine analyses of less complex samples or for preparative chromatography where analyte capacity (loading) is of high importance.
Pore Size
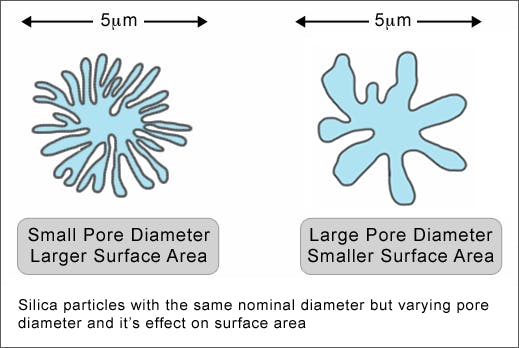
Silica particles for chromatography have a very high surface area, a property that is required for good analyte retention. Most of the surface area is contained in the internal pore structure of the silica particles, which accounts for over 99% of the available surface area. A typical surface area of the silica used for chromatography is around 330m2/g and, in a 150 x 4.6mm column, there may be as much as 1.5 g of silica — meaning your everyday HPLC column has around the same surface area as an average tennis court!
The surface area of the particle is inversely proportional to the pore diameter; therefore, a 3 mm particle with a 120 nm pore diameter will have more than twice the surface area of a 3µm particle with a 300 nm pore diameter.
There are a number of pore diameters used by manufacturers to control retention.
Columns with pore diameters in the range 8 – 12 nm are typically used for the analysis of ‘small molecules’ (< 3,000 Da) which can easily penetrate into the pores and access a great majority of the silica surface. These are known as ‘small or narrow pore’ columns and are not useful for the analysis of larger molecules such as peptides or proteins, which are excluded from the pore due to their larger hydrodynamic volume. Typically, the pore diameter needs to be three times the hydrodynamic diameter of the analyte in order to be accessible. Most manufacturers tend to keep a similar pore volume across columns of the same bonded phase but different particle diameters. When choosing between columns for small molecule analysis, pore diameter is not usually a primary consideration. However, if slightly more or less retention is required, and all other factors have been explored, changing pore diameter can bring about changes in retention without altering selectivity (providing the same bonded phase is used).
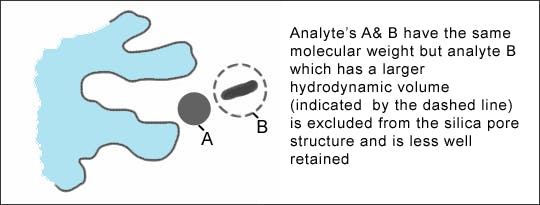
As a general rule, a pore diameter of 10 nm or less should be used for analytes below 3,000 Da. A pore diameter of 10 - 13 Da is recommended for samples in the range of 3,000 - 10,000 Da. For samples above 10,000 Da, including peptides and proteins, a 30 nm material provides the best efficiency and peak shape.