02 Oct 2017
Hydrophilic Interaction Liquid Chromatography – HILIC
The Technical Department at Element are regularly asked for analytical conditions or applications in the HILIC (Hydrophilic Interaction Liquid Chromatography) mode. Here’s a short primer on what you need to understand about this separation mode from a practical perspective, using some of the questions that we regularly get asked:
When can HILIC chromatography give me an advantage?
Typically HILIC is used when retention times in reversed phase mode are insufficient, and this typically involves more polar or ionisable analytes (i.e. where log P values are below 0)
HILIC chromatography can be used as a replacement for Normal Phase chromatography and is particularly useful when using electrospray mass spectrometry as the analyte can be ionised in solution (ensure good ESI efficiency) whilst still showing good retention and the high organic content of the mobile phase ensures efficient droplet desolvation in the API interface of the LCMS.
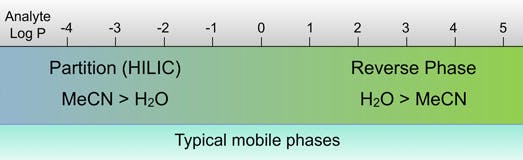
General schema for analyte Log P value versus separation mode of choice
(Adapted with permission from SIELC Technologies, Prospect Heights, Illinois, USA)
So how does HILIC mode work?
The exact mechanism of HILIC chromatography is still subject to a certain amount of speculation, however most experts agree that the bulk mechanism involves polar analyte partitioning into and out of a layer of water which is adsorbed (associated) onto the surface of the polar stationary phase. Although it is also widely understood that as well as this liquid/liquid partitioning behaviour, dipole – dipole and electrostatic interactions are also involved, especially where the eluent pH is adjusted so that the stationary phase surface is charged – predominantly via the anionic surface silanol species at pH >5.
Mobile phases for HILIC chromatography contain a high degree of organic solvent and a typical gradient in HILIC would involve altering the aqueous composition between 5 and 30%.
FLASH 1
More highly hydrophilic species (purple) partition into the water rich layer at the stationary phase surface. Interaction at the surface occurs through dipole-dipole and hydrogen bonding between the surface's functional groups and polar functional groups of the analyte.
FLASH 2
With an unbonded silica surface, and the eluent at pH ~ 5-9 and <~20mM buffer, the surface silanol species will be ionized and therefore able to undergo electrostatic interaction with cations (bases) in solution. Note that the surface will now be less attractive to anions (acids) in solution through electrostatic repulsion. Unless there is also an analyte cation moiety, retention of acids can decrease relative to a separation carried out at an eluent of pH<<5.
What are the best solvents and buffers to use for HILIC separations?
It is generally accepted that the organic eluent content in HILIC mode is 70% or greater. The most popular weak solvent is acetonitrile, due to its aprotic (intermediate polarity, lacking an acidic proton) characteristic which encourages retention of polar analytes. Other ‘weak’ solvents may be used in HILIC mode and the general solvent strength series for HILIC separations is:

Acetone can be used in place of acetonitrile where extra retention is required or there is a need to reduce the amount of acetonitrile. However, it should be noted that the selectivity of the separation will be different and as acetone has a UV cut-off of 330nm it is generally unsuitable for UV detection.
Most MS and evaporative light scattering detectors can be successfully used with acetone as the organic solvent if the detector operating parameters are optimized.
The protic solvents (methanol, ethanol, isopropanol etc.) tend to result in much shorter retention times, as the polar nature of the solvent results in increased competition for polar stationary phase sites and a disruption of the adsorbed water layer.
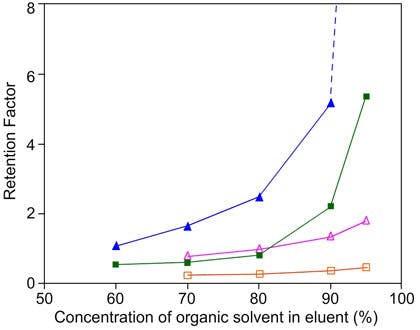
Retention factor as a function of acetonitrile concentration (closed symbols) or methanol (open symbols) cytosine(triangles) adenine (squares). (Reproduced with permission from Merck Sequant, Umeå, Sweden)
Changes in eluent buffer ion concentration have a profound effect on retention in HILIC chromatography due to their influence on the degree of analyte and stationary phase ionization and the polarity of the eluent. Having a sufficiently high ionic strength counter ion is often essential to achieving good peak shape and satisfactory / reproducible retention in the HILIC mode.
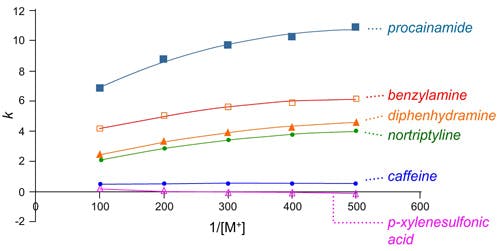
Plots of retention factor vs. 1/[counter-ion concentration] for silica HILIC columns.
Mobile phase: ACN–water (90:10, v/v) containing ammonium formate (concentration varied from 2 to 10 mM) pH 3.0.
Ammonium formate and acetate are popular buffers for use in HILIC separations as they are readily soluble (20mM is typical for HILIC separations), in highly organic solvent systems and are volatile enough to permit their use with MS detection. Crucially, they also provide important counter ions necessary for good peak shape in many HILIC separations, through improved kinetics of diffusion and surface interactions.
Can you recommend a good column to use for HILIC separations?
Bare silica, diol and amide phases are arguably the most popular for HILIC chromatography. However it should be noted that for more refined separations, in which the pH of the analyte or stationary phase can be used as a method development variable to alter retention and selectivity of the separation, ligands containing ionisable moieties are preferred. A range of ligands used for HILIC and so called mixed mode chromatography are shown below.
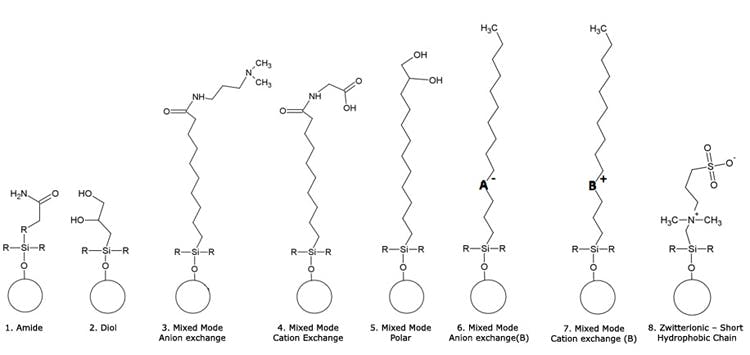
- 1 &2 – Generic structures for Amide and Diol phases (or analogues) offered by a variety of manufacturers
- 3, 4 & 5 – Mixed-mode column structures with terminal functional chemistry based on the Acclaim® column series from Dionex (Sunnyvale, California UA)
- 6 ,7 – HILIC Mixed-mode columns with embedded functional chemistry based on the Primesep® series from SILEC Technologies (Prospect Heights, Illinois, USA)
- 8 – Zwitterionic phase based on ZIC HILIC® (MerckKGaA, Darmstadt, Germany)
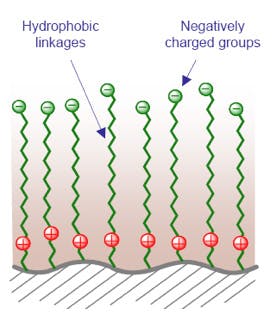
9. Zwitterionic – Long Hydrophobic Chain
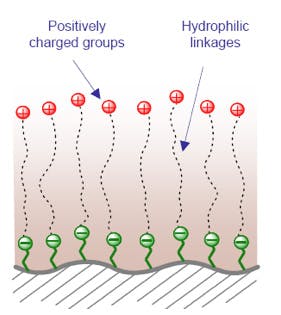
10. Zwitterionic – Long Hydrophilic Chain
9 & 10 – Mixed-mode zwitterionic columns - zwitterionic phases based on the Obelisk® series from SILEC Technologies (Prospect Heights, Illinois, USA), typically used with more conventional reversed phase solvent systems.