20 Aug 2020
Reducing non-specific protein binding in HPLC
When working with biomolecules such as proteins non-specific binding of analytes can occur at any surface within the HPLC flow path. This can cause a range of problems such as reduced sensitivity, peak tailing, carryover effects and reproducibility problems [1]. Whilst it can often also be an issue for small molecules, the binding to is usually mediated by either ionic or hydrophobic/polar interactions. Since one mechanism dominates it is usually straightforward to address. In the case of small molecules two approaches are generally taken; either the pH can be changed (to reduce ionic interactions) or the percentage of organic modifier can be increased (to reduce hydrophobic interactions). Unfortunately, when dealing with biomolecules such as protein, the situation is often more complicated and may require more careful optimization.
If you are having issues with “sticky” proteins the problem will usually get worse with each successive injection as the molecules accumulate in the system [1]. Common indicators that there might be an issue with biomolecules fouling your system are:
- poor peak shape / sensitivity
- spikes in pressure during injection
- reduced flow rates due to increasing back pressure
- ghost peaks from carryover
- frequent clogging of the system
- column damage
This article looks at some tips to help avoid these pitfalls when working with protein analytes. Reducing non-specific binding will help to improve your chromatography, increase column longevity, and decrease instrument down time.
Mechanisms of non-specific protein binding
Due to their complex surface chemistry and amphoteric nature, biomolecules may have multiple affinity mechanisms by which they are adsorbed onto surfaces they meet. This can be challenging as proteins can accumulate and foul your column and instrument. Non-specific adherence of protein analytes onto surfaces can occur due to the complexity of such analytes. Each protein molecule usually has numerous positive and negative charges as well as numerous hydrophobic moieties. Hence, there is the potential for numerous points of interaction that result in protein sticking to surfaces [1]. In addition to carryover and instrument problems, this can negatively impact your chromatography. Slow secondary ionic interactions between the protein and the column can cause problematic peak tailing (Figure 1).
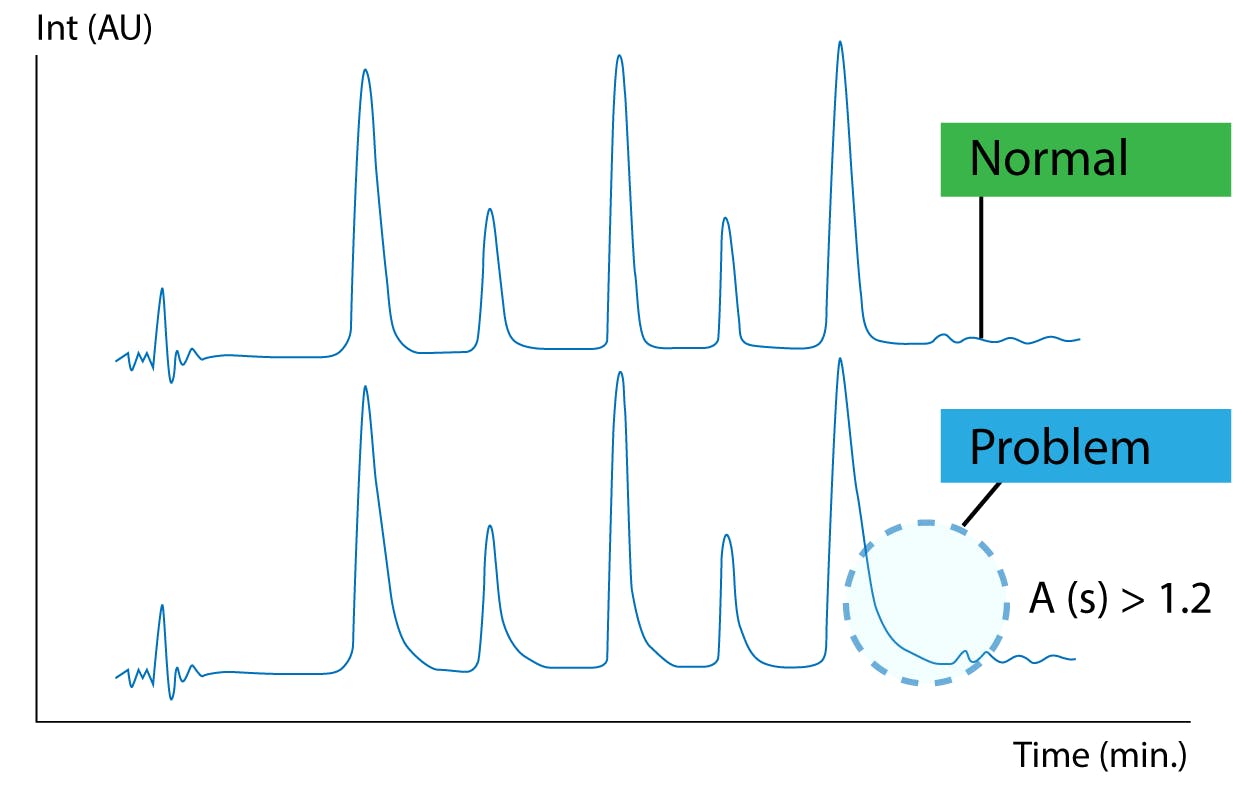
Figure 1 Peak tailing is often seen for biomolecules due to slow secondary ionic interactions [2]
Method considerations
The addition of an ion pairing reagent such as trifluroacetic acid (TFA) is often used to improve peak shapes when working with biomolecules. Since TFA is a strong acid the low pH ensures that the acidic silanol groups on the surface of the silica are fully protonated. TFA can also ion-pair with the positive charges on the protein. This combined effect is usually sufficient to reduce the undesired ionic interactions between the protein and the silica column. Although additives can be useful for improving peak shape, it is still important to choose a column optimized for working with proteins and large molecules. These columns will generally have a larger pore size to allow the protein better access to the stationary phase and will provide limited access to residual silanols (i.e. through end-capping of using a hybrid silica material). However, for particularly “sticky” proteins you may need to consider moving to a polymer column where the problem of silanol interactions is avoided completely [2].
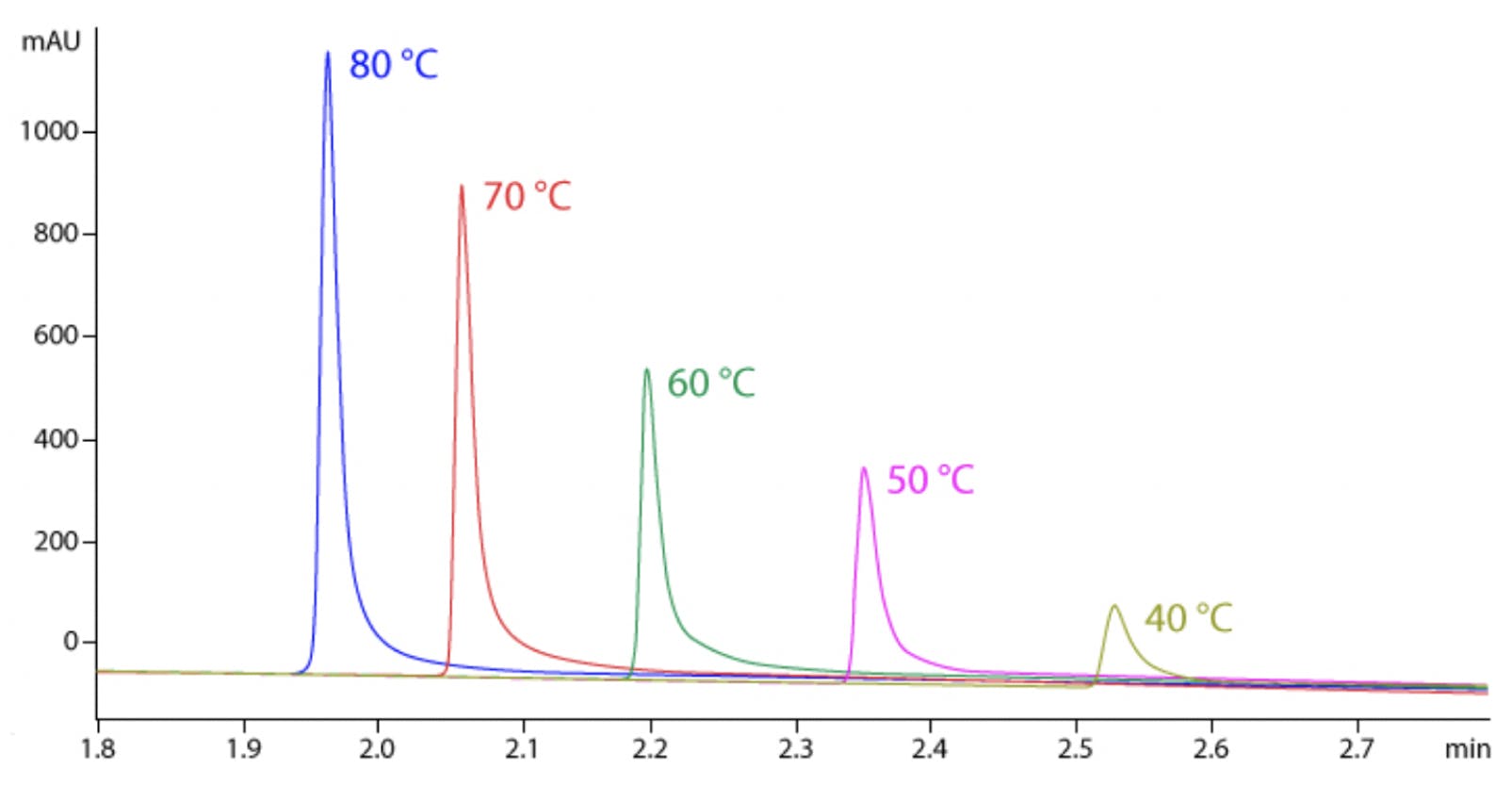
Figure 2 Intact mAb analysis at 40-80 °C [2].
Although high temperatures are not routinely used for small molecule analysis, they can be advantageous when working with proteins since this will speed up the kinetics of the secondary interactions and minimise adsorption as shown in Figure 2 [4]. Since large molecules are often sensitive to extreme temperature, it is usually necessary to keep the length of the method short to prevent thermal degradation. Problems with sample stability can usually be identified by spurious ghost peaks and sample to sample variation as shown in Figure 3 [4]. Another downside of working at higher temperature to consider is that elevated temperatures will generally decrease column longevity and increase column bleed, so choosing a column that is robust enough to give satisfactory column lifetimes at these higher temperatures is essential.
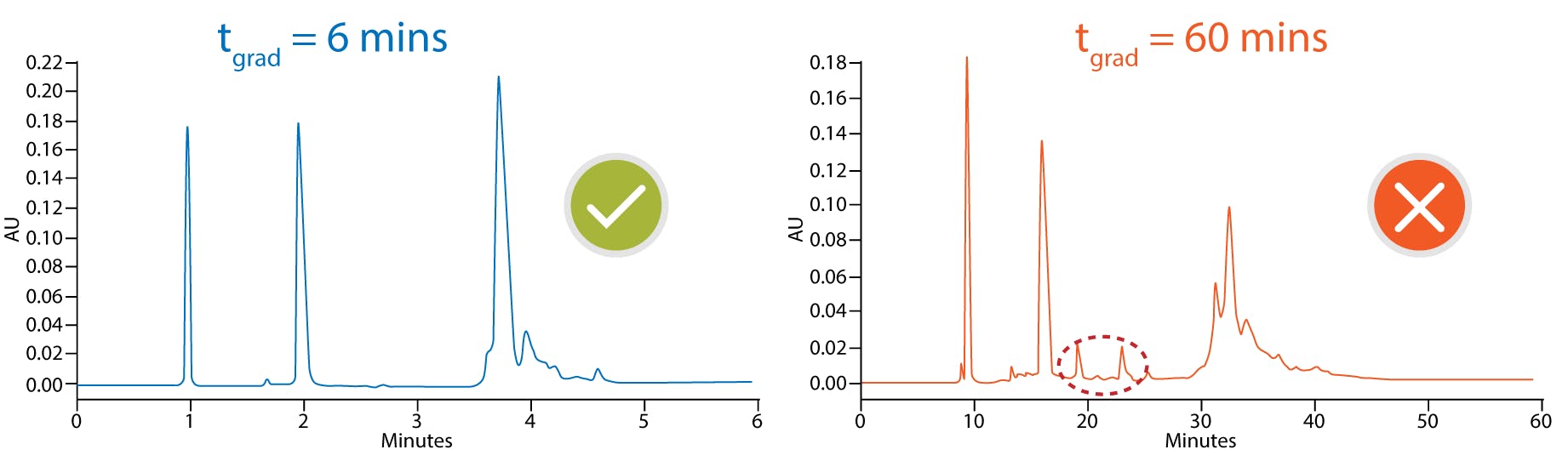
Figure 3 mAb fragment time dependent on-column thermal degradation at elevated temperatures. 150 x 2.1 mm, 3.6 µm SPP C18, 90 °C, FLD 280-360 nm [2]
mobile phase A: 0.05% TFA
mobile phase B: 0.05% TFA in acetonitrile
gradient 30-75%B 6 min flow 300 µL/min (blue)
gradient 30-37%B 60 min flow 30 µL/min (red)
Bioinert flow paths
Ensuring the HPLC flow path is inert is just as important as selecting the correct stationary phase and method conditions. Stainless steel is commonly used in both HPLC hardware and column housing. Since biomolecules have multiple electron donors, they often have a high affinity for sticking to these metal surfaces.
Columns with PEEK hardware (or other biocompatible materials) are recommended for proteins where metal binding is of particularly concern [3]. Choosing a “bio-inert” or “bio-compatible” HPLC system can also reduce problems [2]. However, where this is not possible regular passivation of the system and use of a suitable chelating agent can help to reduce metal mediated binding. It is also possible to make a standard HPLC flow path more bio-inert with some simple changes, for example swapping to PEEK tubing and fittings.
Even when using bioinert systems and columns, a certain level of system priming can be required to reduce the accumulation of biomolecules, particularly when working with ion exchange or size exclusion chromatography methods [2]. The aim of priming the system is to coat the surfaces with something other than your analyte of interest (i.e. a blocking agent). This works because the blocking agent occupies the active sites so that the surface is no longer free for the analytes of interest to bind, thus preventing adsorption of the proteins of interest. Common blocking agents are usually sacrificial proteins such as bovine serum albumin (BSA), casein and plasma. Typically, analysts inject the protein multiple times to prepare the system for consistent performance. Multiple injections are needed to reach a steady state where the active sites have been filled with the surrogate protein, so that when the sample of interest is injected the peak area and analyte recovery are consistent between runs [2].
Beyond HPLC
Using containers and flow paths with inert surfaces can help to mitigate binding of biomolecules. When considering small molecules, choosing deactivated (or silanized) glass containers over glass with a high surface silanol activity, is generally enough to mitigate binding of moderately basic analytes [4]. However, this is not usually enough for biomolecules and it is often necessary to use polypropylene containers to prevent ionic interactions. Note that care still needs to be taken when using polypropylene as this hydrocarbon rich material can sometimes actually promote binding of very hydrophobic proteins. Therefore, choosing a high quality and low-binding polypropylene, is needed to ensure the highest recovery of such analytes [5].
Non-specific binding of proteins onto surfaces does not occur instantly upon contact with a surface. The longer a peptide or protein is stored in solution the more analyte binding can occur and the lower the recovery. Temperature also influences the kinetics of this binding and influences the extent that it occurs, with adsorption occurring fastest at higher temperatures [5]. The analyte concentration and sample volume will also influence analyte recovery. Each surface has a finite number of active sites, therefore for the same vial a similar amount of analyte will be lost regardless of the sample and will cause the biggest problems for low volume and low concentration samples. Although there are various “low-binding” innovations available, such as 96-well plates that have been plasma treated, each individual biomolecule will have a different affinity for these surfaces so it is important to evaluate recovery and find the polypropylene vials or well plates that give the maximum recovery for the analyte of interest. This is particularly important when trying to detect low abundance analytes by LC-MS [5].
Summary
When working with biomolecules it is important to consider every surface in the sample preparation and HPLC flow path. Recovery issues can start even prior to injection to the HPLC so both sample preparation and the HPLC flow path need to be considered when trying to measure low concentrations of analyte. At Element we have experience helping many of our customers overcome problems related to protein analysis. If you are experiencing issues contact our technical team for advice on your specific method ([email protected]).
References
- https://www.silcotek.com/blog/identify-and-prevent-fouling-in-hplc-systems
- https://www.chromacademy.com/chromatography-Reversed-Phase-Biomolecule-Analysis.html
- http://www.chromatographyonline.com/bioinert-versus-biocompatible-benefits-different-column-materials-liquid-chromatography-separations
- https://www.chromacademy.com/channels/bio-chromatography/technique/reversed-phase-protein-level-analysis-and-optimization
- http://www.chromatographyonline.com/strategies-improve-recoveries-proteins-and-peptides-sample-containers-lc-ms-analyses