21 Sep 2020
Released Glycan Analysis
Introduction
Glycoproteins are proteins that contain complex polysaccharide chains, called glycans, bonded to their structure. The presence of glycans increases the solubility hydrophilicity or the protein, and affects its specific tertiary structure. Glycoproteins have a wide range of functions in the organism: certain hormones (EPO, HCG), immunoglobulins (antibodies), thrombin, prothrombin, fibrinogen, collagen or mucins are all glycoproteins.
Glycans affect the biological activity, stability, clearance, immunogenicity, and pharmacokinetics of therapeutic glycoproteins. Not surprisingly, one of the most important critical quality attributes (CQA) for biologicals such as monoclonal antibodies (mAbs) is glycosylation: determining the presence, number, and identity of glycan structures attached to the protein.
The most important class of glycans, called N-linked glycans, are covalently bonded to the amidic side chain of asparagine amino acids; specifically those in a “Asn-X-Ser” or a “Asn-X-Thr” sequence, where X is any amino acid except proline. In human IgG, this amino acid is asparagine 297 (Asn297).
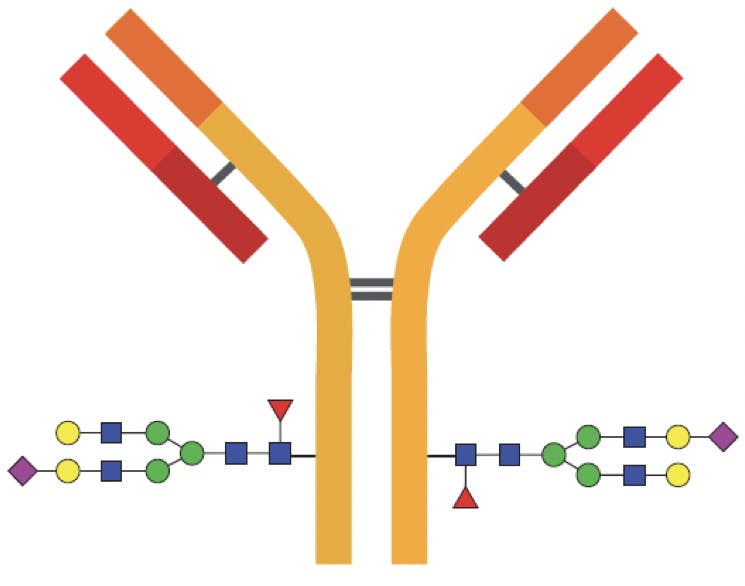
Figure 1: Diagram of an IgG indicating the most common glycosylation sites on the Fc region. In some cases the Fab regions are also glycosylated.
Some glycoproteins have a different class of glycans, named O-linked glycans because they are bound to the hydroxyl groups of serine or threonine through an O-glycosidic bond. O-glycans can be found in the Fab region of certain antibodies, but their structures are less predictable than N-glycans and their characterisation requires a unique workflow.
Glycan Structure
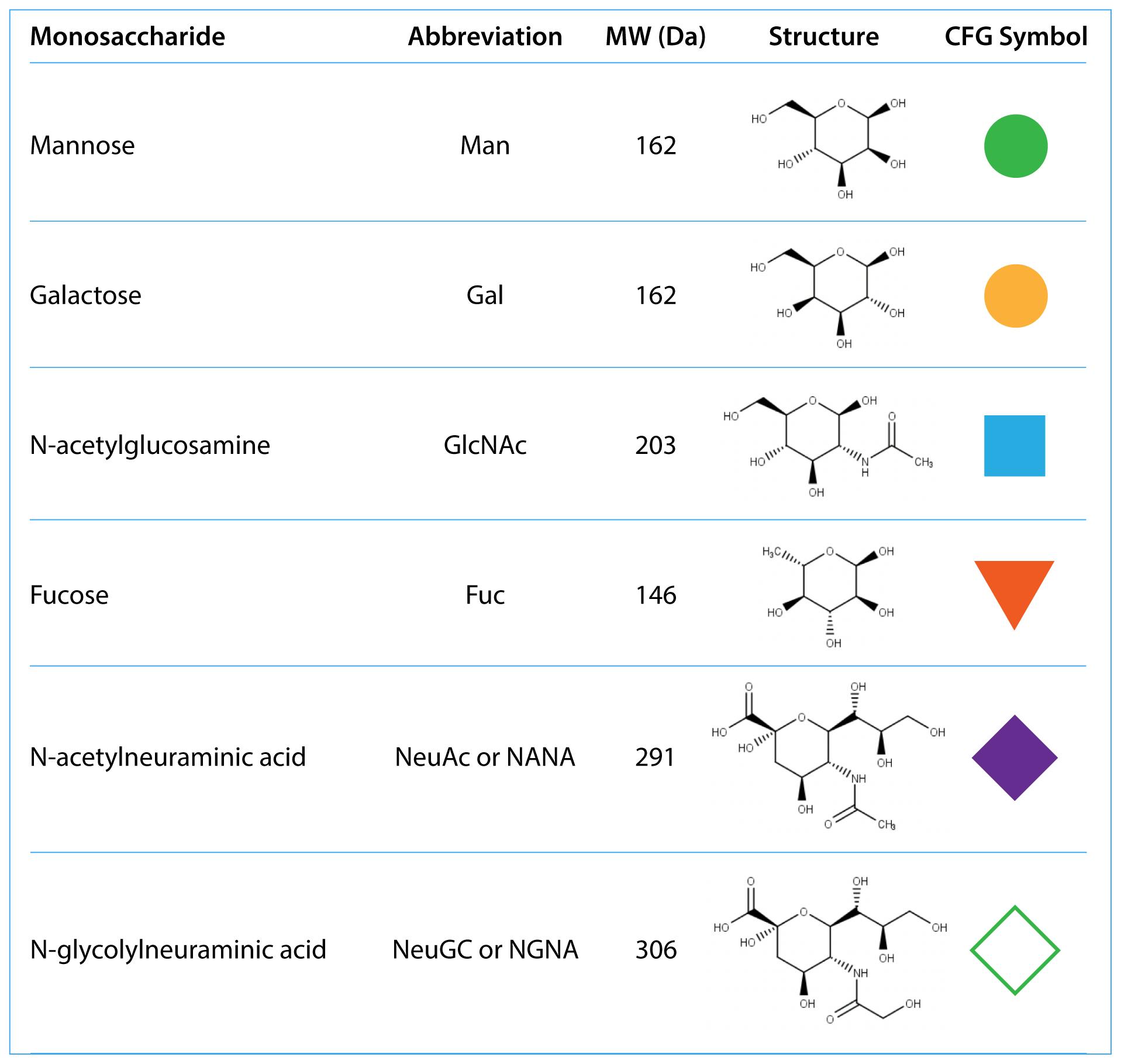
Glycans are complex, highly branched structures of monosaccharides. The most common monosaccharides are N-acetylglucosamine, mannose, galactose, fucose and sialic acids:
Table 1: Common monosaccharides found in mAbs. CFG stands for Consortium of Functional Glycomics.
All N-linked glycans have a common core structure, a pentasaccharide constituted by two N-acetylglucosamine (GlcNAc), called the chitobiose core, and three mannose (Man) in the following arrangement:
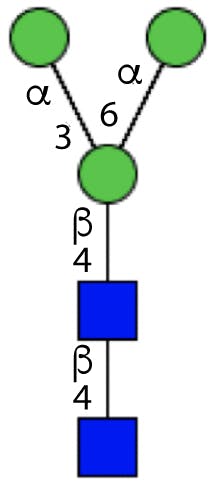
Figure 2: Glycan core structure.
The terminal GlcNAc is bonded to the Asn residue on the protein through the N-glycosidic bond.
Additional branches can be attached to the mannose monosaccharides, resulting in complex structures. Depending on the composition of the branches, glycans are classified as:
- High mannose glycans, which contain between five and nine mannose sugars, but no other monosaccharides.
- Hybrid glycans, which contain substituted and unsubstituted mannose branches. The substitutions (called antennae) are additional GlcNAcs, with potentially other sugars.
- Complex glycans, which do not contain mannose beyond the core, and have heavily substituted antennae.
Hybrid and complex glycans can be bisected (having an additional GlcNAc attached to the middle core mannose), sialylated (with terminal sialic acids), and fucosylated at the innermost GlcNAc.
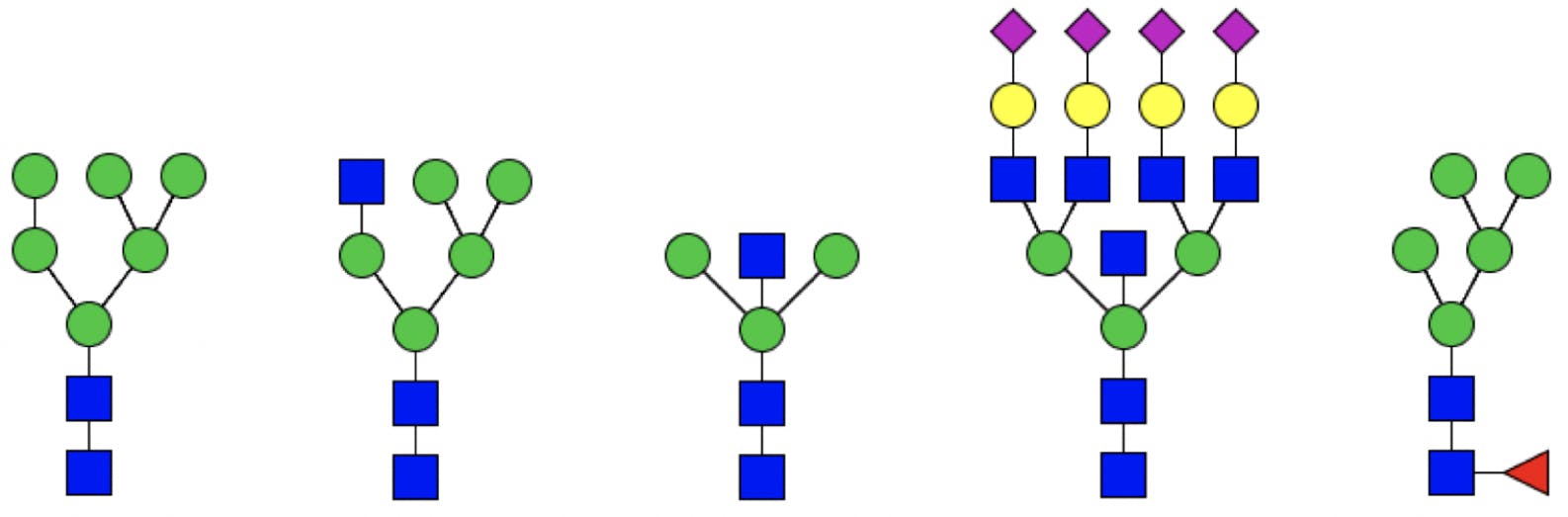
Figure 3: Different types of glycans. From left to right: high mannose, hybrid, bisected, complex (tetra antennary), fucosylated.
Since the core structure of N-linked glycans is preserved, an abbreviated nomenclature system is possible, which only needs to indicate the terminal residues attached to the core.
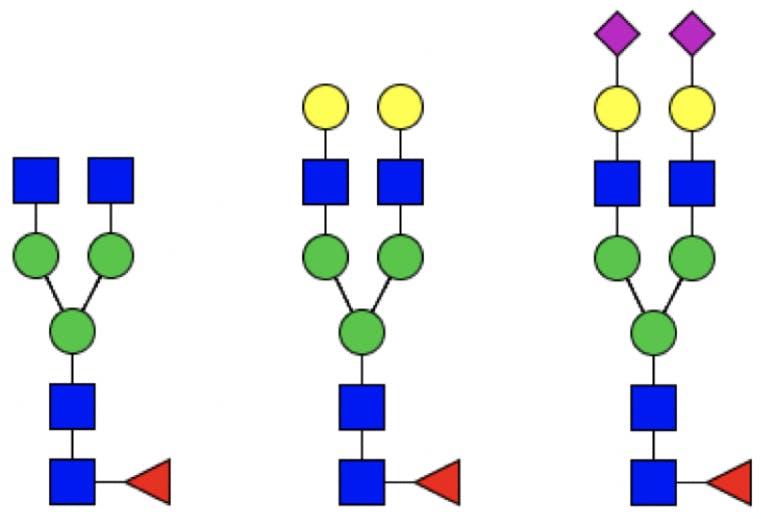
Figure 4: From left to right: G0F, G2F, G2FS2.
Where Do Glycans Come From?
Proteins are synthesised in the cell by ribosomes, in a process called “translation”: DNA from the cell nucleus is transcribed into messenger RNA (mRNA) and released to the cytoplasm. Ribosomes in the cytoplasm “read” mRNA and “translate” it to a sequence of amino acids, the primary structure of the protein.
Proteins destined to be secreted outside the cell, such as antibodies, are synthesised by ribosomes associated to the endoplasmic reticulum (ER), a large organelle surrounding the cell nucleus. As the peptide grows, it is transported (translocated) to the interior of the ER, where it undergoes a series of post-translational modifications (PTMs).
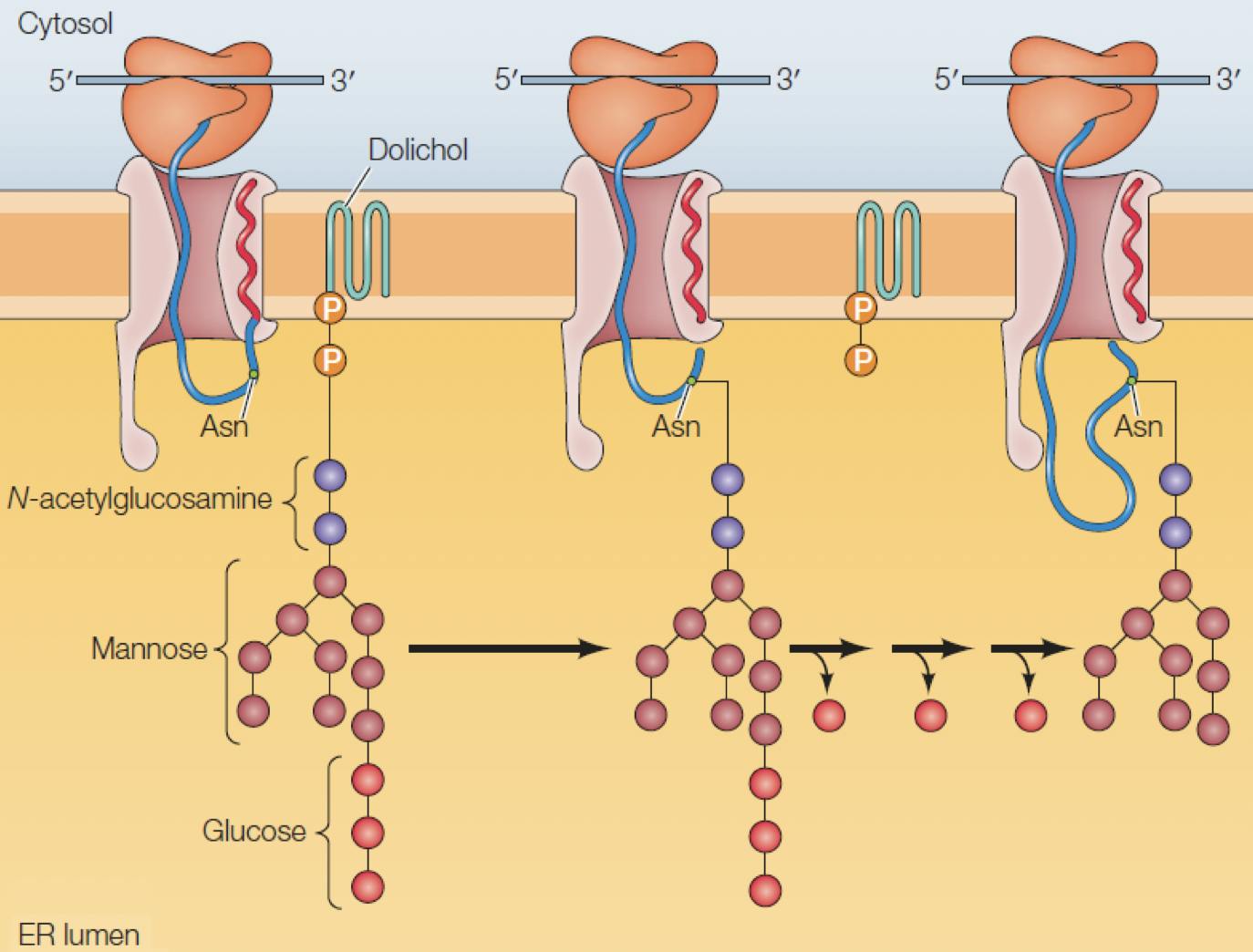
Figure 5: A peptide being translated from mRNA by a ribosome is translocated into the ER, where a new glycan is attached to an asparagine residue. Adapted from Cooper, 2019
Glycosylation is one of the first and most important PTMs to occur. A glycan consisting of two GlcNac, nine Man, and three glucose, is anchored to the ER membrane, close to the translocation pore. If a “Asn-X-Ser/Thr” sequence is detected in the peptide, an enzyme binds the glycan to the asparagine, resulting in an N-glycosidic bond:
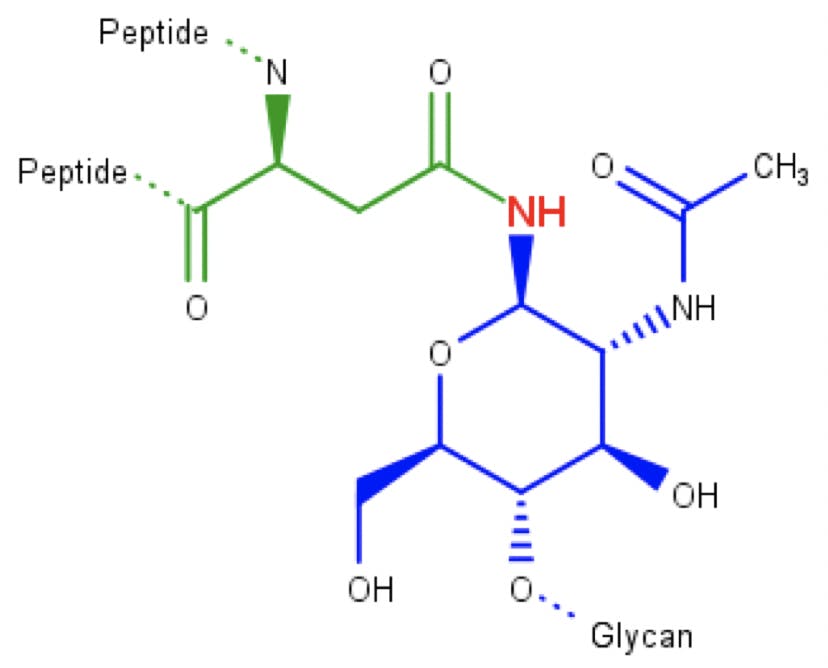
Figure 6: N-acetylglucosamine (blue) attached to an asparagine amino acid (green) through an N-glycosidic bond.
Folding the amino acid sequence into secondary and tertiary structures is the next step in the protein synthesis. This is done inside the ER with the help of so-called molecular chaperones, which gradually cleave the three glucose sugars at the end of the glycan as the protein is folded. These glucose residues seem to work like kanban cards to control folding.
If folded correctly, the glycoprotein will be transported to the Golgi apparatus for further PTMs, which may include cleaving and adding additional monosaccharides to the glycan. Therefore, all N-glycans begin life as high mannose structures, until they are further modified in the Golgi.
Released Glycan Analysis
Released glycan analysis is the gold standard for accurate identification and relative quantitation of N-linked glycans. The workflow, illustrated below, involves six major steps:

- The glycoprotein is denatured to unravel its tertiary structure (sometimes also cleaved into peptides, depending on the protocol being used) and make glycans more accessible.
- The glycans are separated from the protein or peptide by chemical treatment (e.g. hydrazinolysis) or by means of a peptide N-glycosidase (PNGase) enzyme, which selectively cleaves the N-glycosidic bond. Enzymatic deglycosylation has superseded chemical methods.
- A purification step is performed after deglycosylation to prepare the glycans for labelling. Traditional reductive amination labelling chemistries require an additional “finishing” step.
- Since glycans do not have chromophores, they must be “labelled” to be detected. Labels, or tags, are small molecules with a fluorophore for fluorescence detection, or an ionisable group for MS detection. Two alternative reaction mechanisms exist, discussed below.
- Excess label and reagents are removed to minimise unwanted side reactions and background interference.
- The labelled glycans are ready for analysis by high performance liquid chromatography (HPLC) or capillary electrophoresis (CE).
HPLC analysis is generally carried out by HILIC with fluorescence (FLD) or MS(-MS) detection, sometimes in series. FLD is typically used for glycan quantitation, and MS(-MS) is used for mass confirmation and structural elucidation.
Multiply charged fluorescent labels also been developed for capillary electrophoresis analysis (CE-LIF or CE-MS).
About ProZyme…
This has traditionally been a delicate and labour-intensive procedure, requiring two or even three days of careful manual work. However, a number of innovations have been introduced over the last decade that have resulted in a faster and more robust workflow, which can be fully automated and carried out in a fraction of the time. ProZyme Inc., founded in 1990 in Hayward, California, is one of the pioneering companies in glycan analysis behind many of these innovations. The following chart illustrates the evolution of N-glycan analysis products, emphasising the remarkable reduction in sample preparation time:
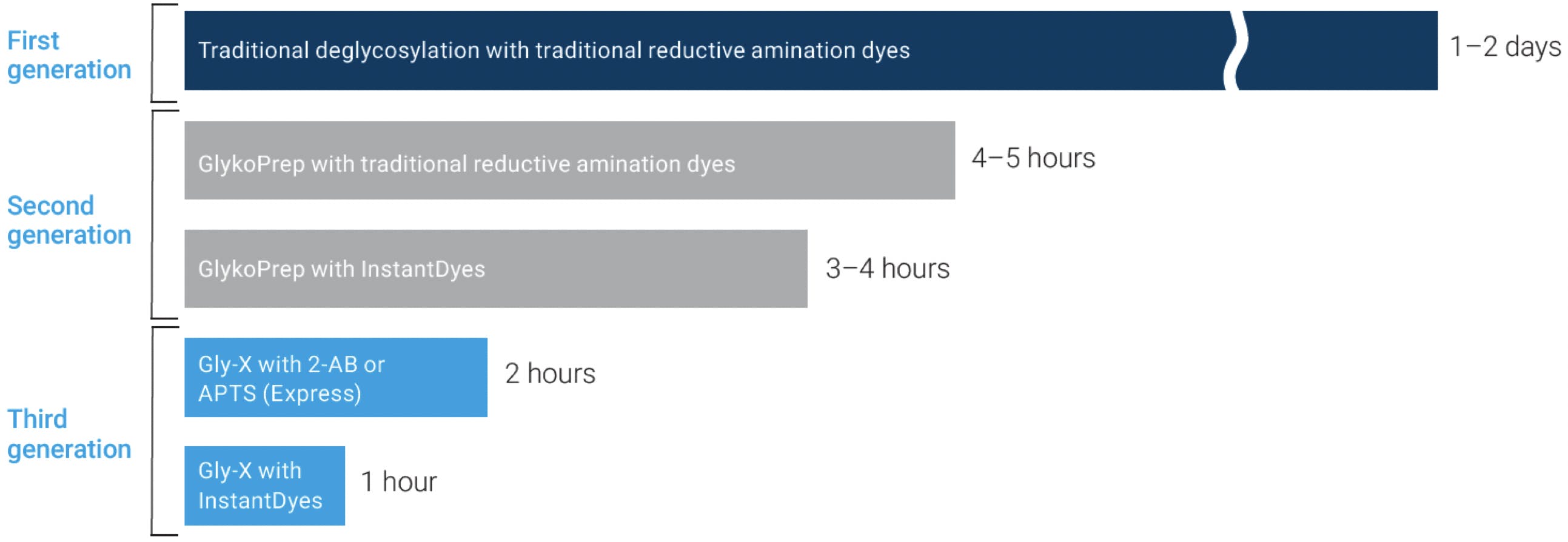
Figure 7: Evolution of Prozyme N-linked glycan analysis products. Adapted from Agilent Technologies.
The latest generation of N-glycan sample preparation kits, Gly-X™ with Rapid Release and InstantPC™ Labelling, incorporates the latest in deglycosylation, labelling and glycan clean-up technology:
Rapid Release deglycosylation incorporates a proprietary surfactant formulation for fast denaturing of glycoproteins, and N-Glycanase (PNGase F for enzymatic deglycosylation) in an optimised buffer. Denaturing takes place in 3 minutes at 90°C, and quantitative deglycosylation is completed in 5 minutes at 50°C.
InstantPC™ is a fluorescent and ionisable tag, with a structure and chemistry quite different from 2-aminobenzamide (2-AB) and similar labels used in traditional glycan analysis:
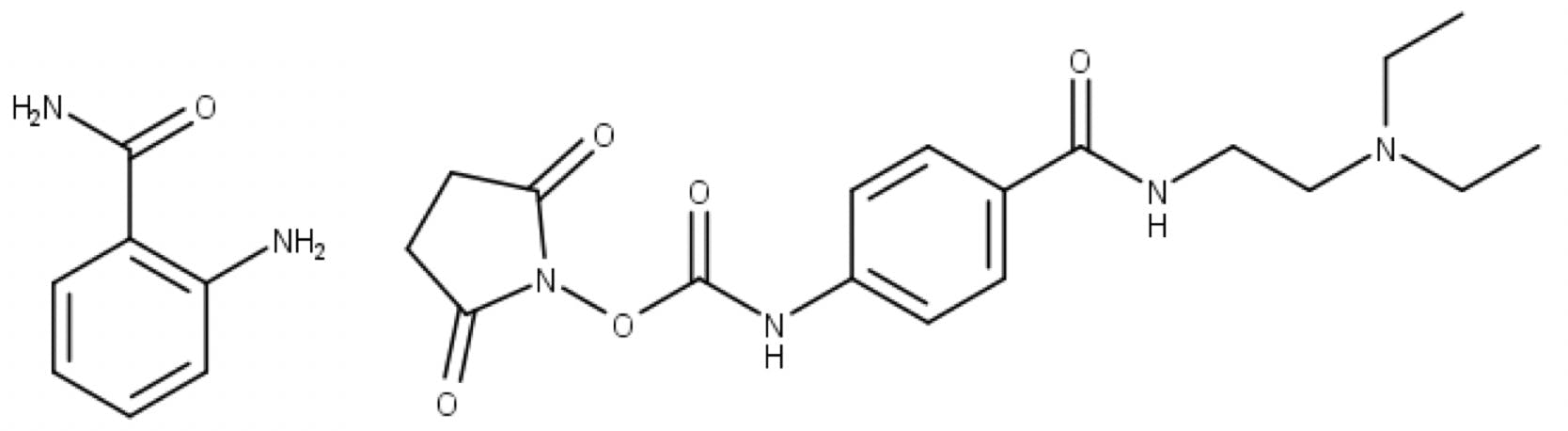
Figure 8: Reductive amination label 2-AB (left) and rapid tagging label InstantPC (right).
InstantPC™ incorporates an N-hydroxy succinimide (NHS) carbamate group (known as an activated carbamate). Unlike traditional labelling, which requires two to four hours of reaction time at 65°C, activated carbamates take just minutes to quantitatively label released glycans, and the reaction can proceed at room temperature. On the Gly-X commercial kit, labelling is done in one minute at 50°C.
Traditional fluorescent labels like 2-AB, aniline, 2-aminobenzoic acid (2-AA), etc., bind to glycans through reductive amination, a two-step reaction. In the first step, the free aldehyde of the sugar reacts with the amine group of the label to form an imine (Schiff base):
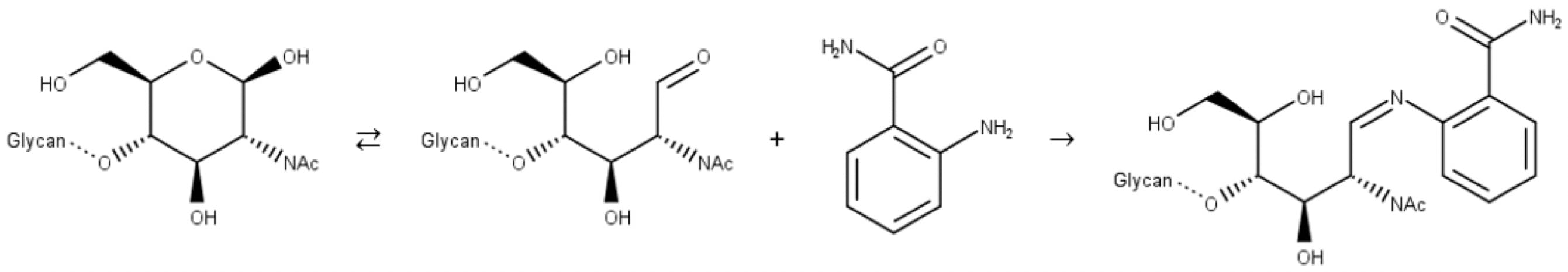
Figure 9: Reductive amination of a glycan. Step 1: imine formation.
In the second step, a reducing agent (most commonly sodium cyanoborohydride) reduces the imine to a secondary amine:
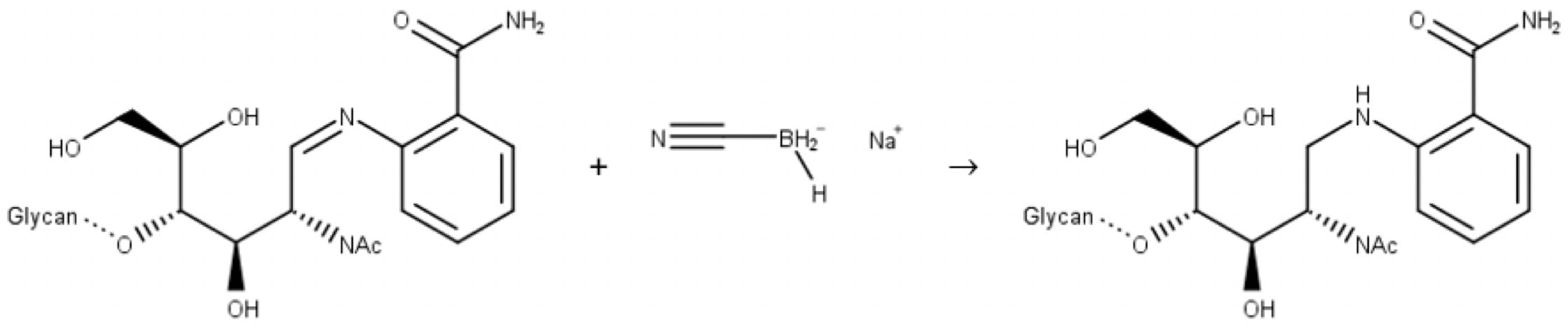
Figure 10: Reductive amination of a glycan. Step 2: imine reduction.
Unfortunately, this reaction has two practical drawbacks: enzymatically deglycosylated glycans are not released with a free aldehyde group, but an amine. They therefore need to undergo a “finishing” hydrolysis step (a minimum of 10 minutes at 50°C) to make them amenable to 2-AB labelling:
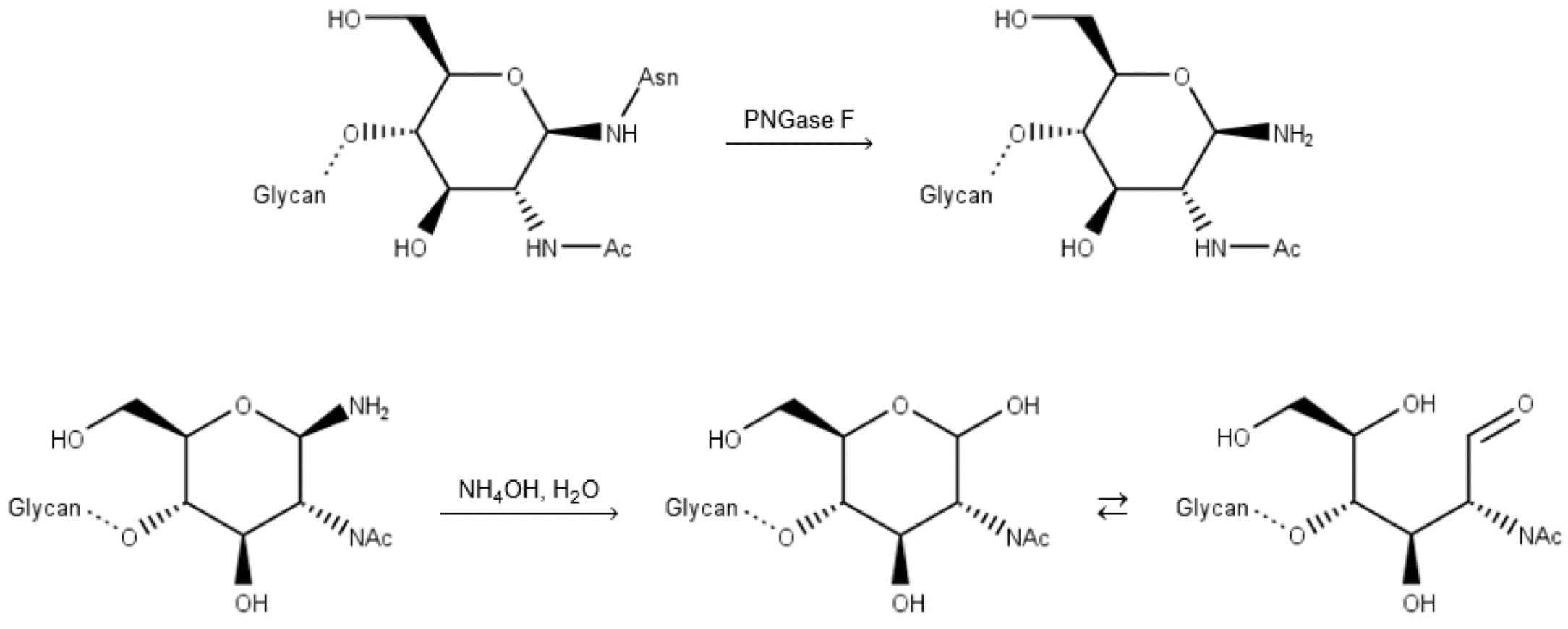
Figure 11: Deglycosylation by PNGase F, followed by basic hydrolysis (finishing) of the free amine.
Furthermore, water must be removed from the finished glycan, because reductive amination requires anhydrous conditions (acidified DMSO, THF). This is routinely done overnight by centrifugal vacuum evaporation at low temperature, becoming one of the most time-consuming operations of the whole workflow.
Contrary to reductive amination labels, activated carbamates react with the amine group of the released glycan, making the “finishing” step unnecessary. What is more, the reaction proceeds in aqueous conditions, eliminating the need for lengthy and unreliable drying stages. This greatly simplifies the protocol, reduces glycan degradation, and improves the reproducibility of the overall method:
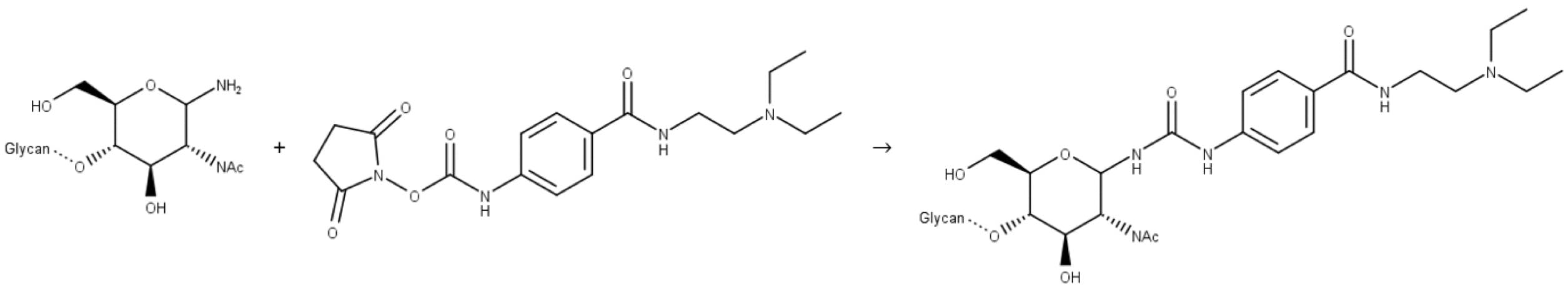
Figure 12: Reaction of a release N-glycan with InstantPC.
One of the main CQA requirements for glycoproteins is relative glycan quantitation, i.e. determining the percentage of each glycan species. Given the stoichiometry of the Schiff base and activated carbamate labelling reactions, each glycan can get only one fluorophore and direct relative quantitation by HPLC-FLD is possible. Because of their different degrees of hydrophobicity, different labels shift the retention times of labelled glycans by different amounts:
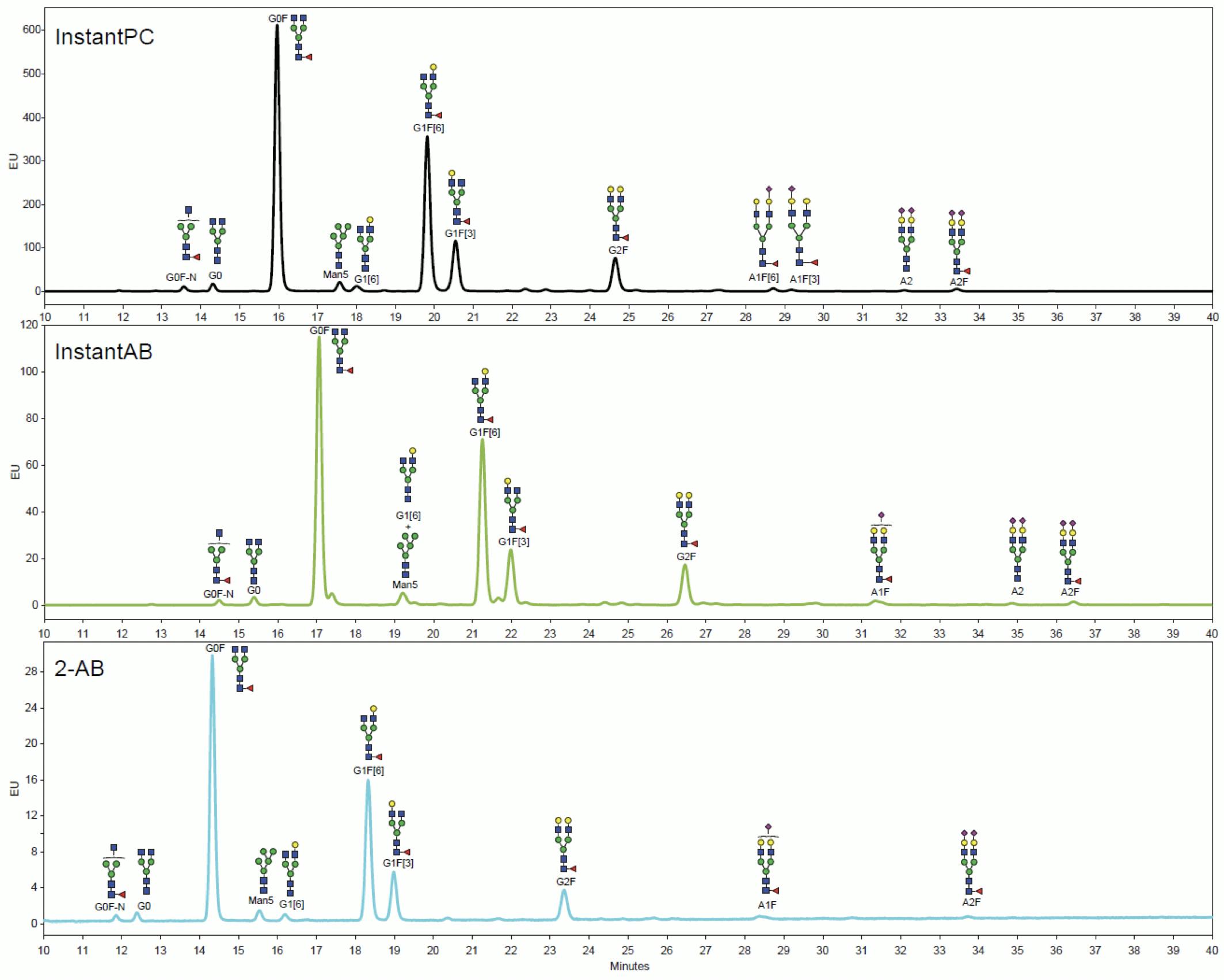
Figure 13: HILIC-FLD chromatograms of equal amounts of MabThera N-glycans labelled with different tags analysed on Agilent AdvanceBio Glycan Mapping 1.8μm 2.1×100mm HILIC column and processed on MassHunter BioConfirm. Adapted from Agilent Technologies.
Relative quantitation by LC-MS can be more complex, because ionisation efficiency depends on the label and the structure of the glycan. InstantPC-labelled glycans have been shown to give similar relative quantitation results for LC-MS and LC-FLD analysis:
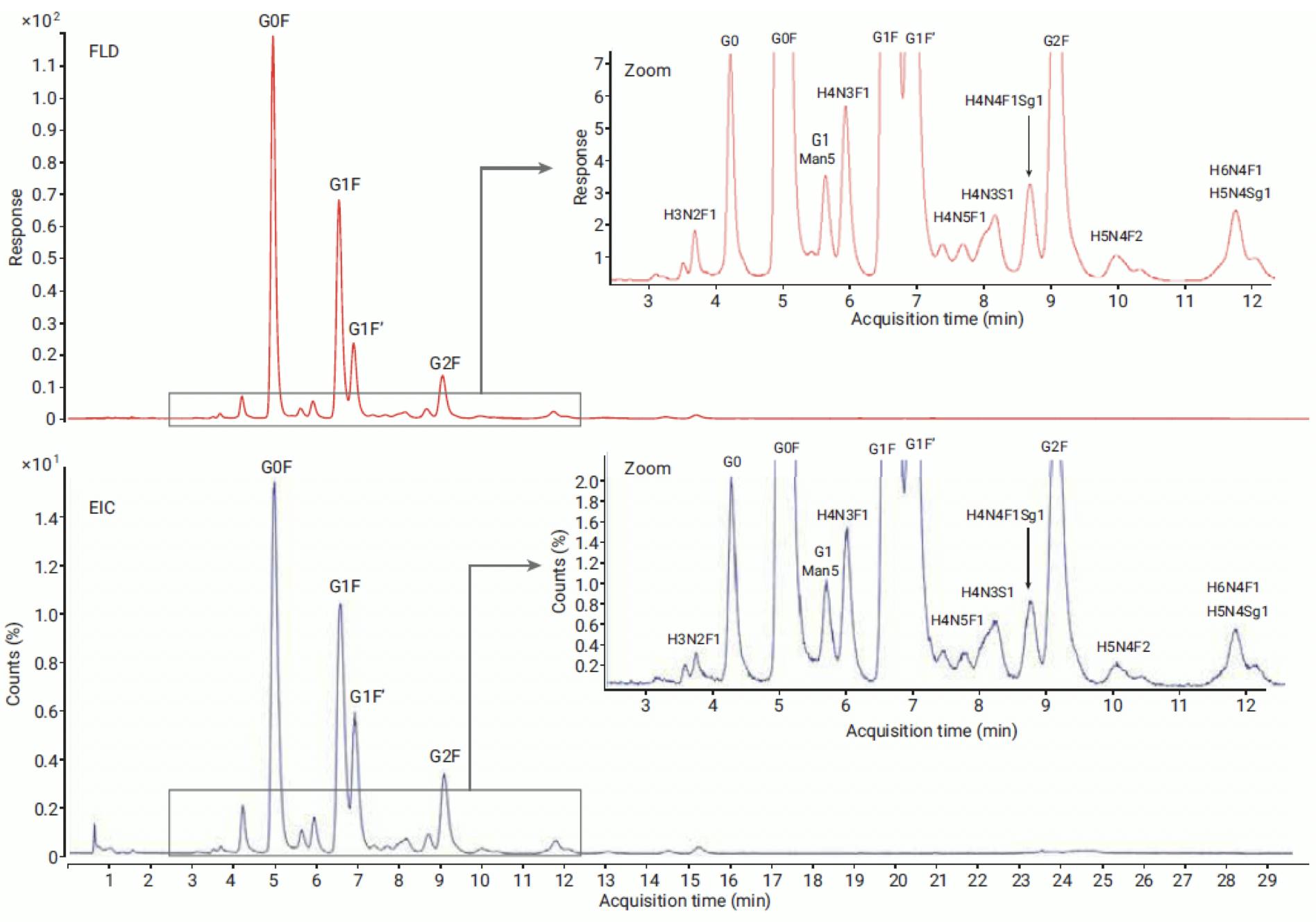
Figure 14: Comparison of FLD (top) and MS EIC (bottom) profile of NISTmAb released glycans labelled with InstantPC and analysed on Agilent AdvanceBio Glycan Mapping 1.8μm 2.1×100mm HILIC column. Glycosylation pattern of major abundant glycans is comparable between FLD and MS data. Reproduced from Agilent Technologies.
Most commonly, however, relative quantitation is performed with FLD detection and MS is used for identification and structural elucidation:
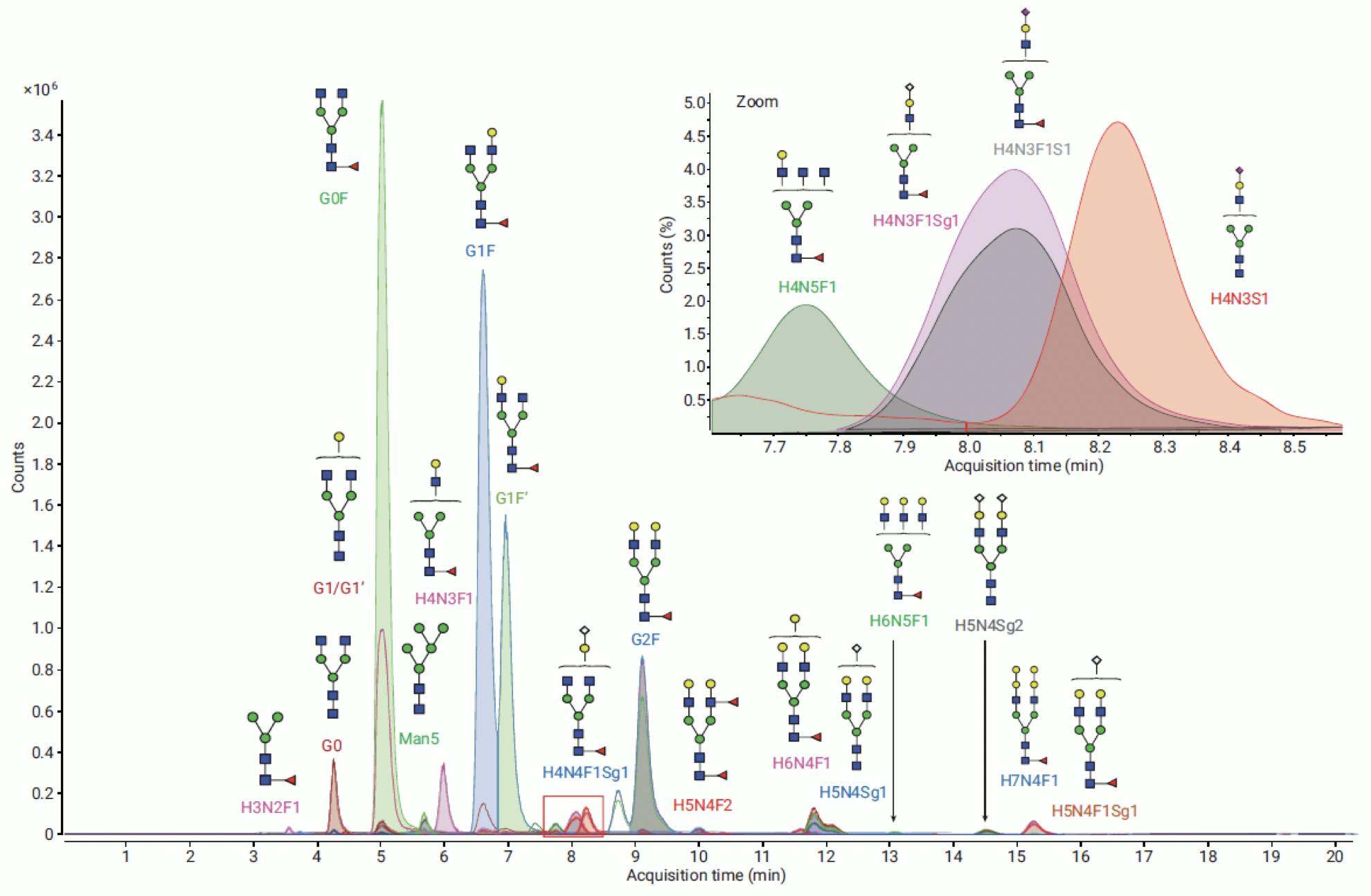
Figure 15: Extracted Ion Chromatograms of glycans from NISTmAb labelled with InstantPC, analysed on Agilent AdvanceBio Glycan Mapping 1.8μm 2.1×100mm HILIC column. Identified against PCD (personal compound database) on MassHunter BioConfirm. Adapted from Agilent Technologies.
… And Element
Glycans are complex polysaccharide structures attached to glycoproteins during biosynthesis, through O-glycosidic and N-glycosidic bonds. These structures are fundamental to the biological activity, toxicity and clearance of glycoproteins, and are therefore critical quality attributes of many biotherapeutics. One of the most accurate techniques to quantify and identify the glycan moiety of glycoproteins is called released glycan analysis, in which glycans are cleaved from the protein and analysed separately. In order to make them amenable to fluorescence and MS detection, released glycans are labelled with fluorescent and MS-active “tags”. The Prozyme Gly-X™ kit illustrated in this article is the state of the art in release, labelling, purification and analysis of N-linked glycans.
ProZyme Inc. was acquired by Agilent Technologies in 2018. The Prozyme (now AdvanceBio) Gly-X™ with Rapid Release and InstantPC™ Labelling kit highlighted here is the flagship product, but the portfolio also includes a complete range of reagents, enzymes, standards, and sample purification kits to cater for any deglycosylation and labelling workflow, including other reductive amination and instant labels.
Combined with AdvanceBio Glycan Mapping HILIC columns, Infinity II HPLC and LC-MS systems, and MassHunter BioConfirm software, Agilent is in an exceptional position to support the entire N-glycans analysis workflow, from sample preparation to structural confirmation.
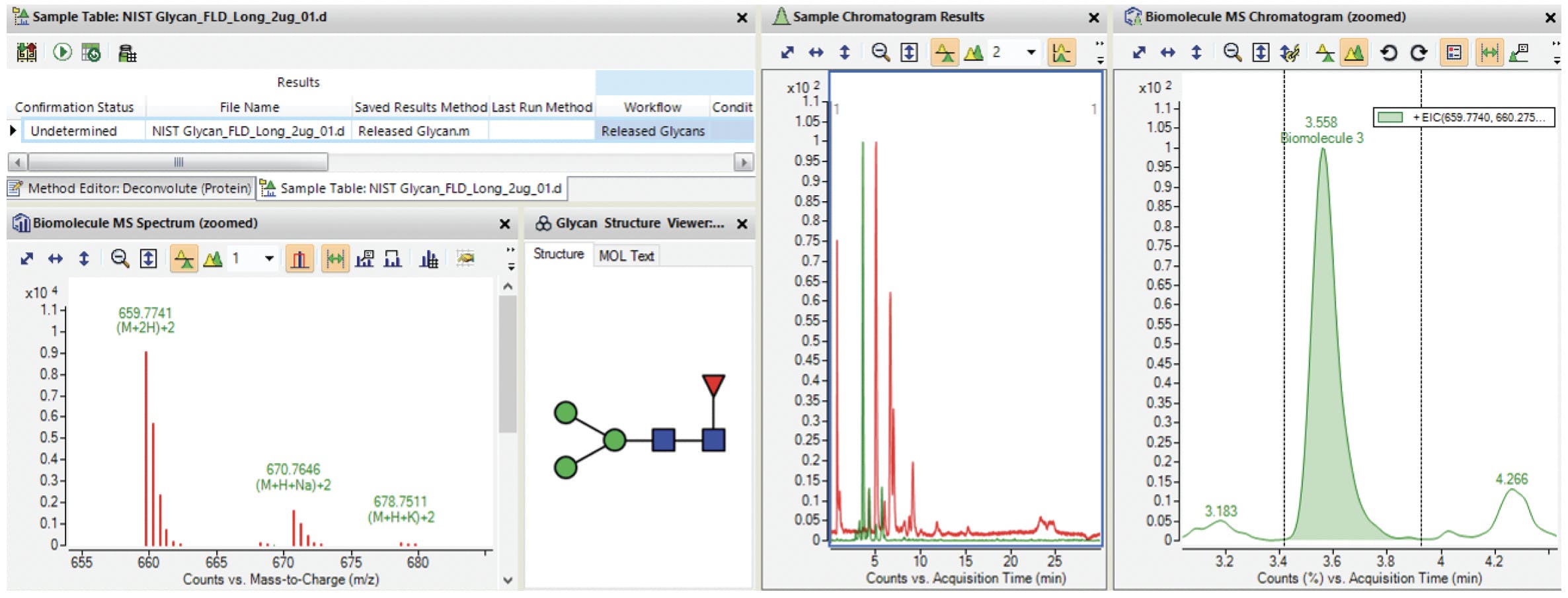
Figure 16: Screenshot of Agilent MassHunter BioConfirm B.09.00 with glycan profiling results. Adapted from Agilent Technologies.
The complete AdvanceBio Glycan range is now available through Element, and we are delighted to support our customers in this demanding and important application field. In addition to our expertise in column chemistry and method development for biotherapeutics, we also bring you the combined expertise of the ARCH Sciences Group in Lab Automation and LC-MS Analysis and Data Interpretation.
References
Consortium for Functional Glycomics, www.functionalglycomics.org
GlycoScience Protocol Online Database, https://jcggdb.jp/GlycoPOD
Cooper, G. M., ‘The Cell. A Molecular Approach’, 8th Ed., Oxford University Press, 2019
Klapoetke, S., Zhang, J., Becht, S., Gu, X., Ding, X., ‘The evaluation of a novel approach for the profiling and identification of N-linked glycan with a procainamide tag by HPLC with fluorescent and mass spectrometric detection’, J Pharm Biomed Anal 2010, 53 (3), 315-24
Cook, K. S.; Bullock, K.; Sullivan, T., ‘Development and qualification of an antibody rapid deglycosylation method’, Biologicals 2012, 40 (2), 109-17
5991-7813EN, Precise Characterization of Intact Monoclonal Antibodies by the Agilent 6545XT AdvanceBio LC/Q-TOF, Agilent Technologies, 2017
5991-8550EN, A Comprehensive Approach for Monoclonal Antibody N-linked Glycan Analysis from Sample Preparation to Data Analysis, Agilent Technologies, 2017