18 Sep 2017
Peak Tailing in HPLC
Peak tailing is the most common chromatographic peak shape distortion. We want to address how to go about fixing these distortions but first, let's understand what causes peak tailing.
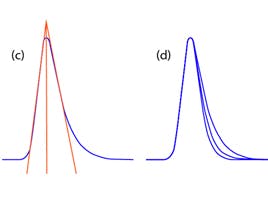
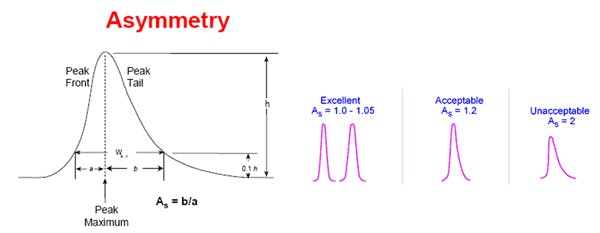
Peak tailing occurs when the peak asymmetry factor (As) is greater than 1.2 — although peaks with As greater than 1.5 are acceptable for many assays. This is determined using the following equation:
As = B / A; where B = peak width after the peak centre at 10% peak height; and A = peak width at baseline before the peak centre,
Get ongoing technical support as a Element columns and consumables customer - the UK's premier supplier
As well as ongoing technical support, benefit from fast delivery, great after sales care, while shopping from over 100,000 products from leading manufacturers
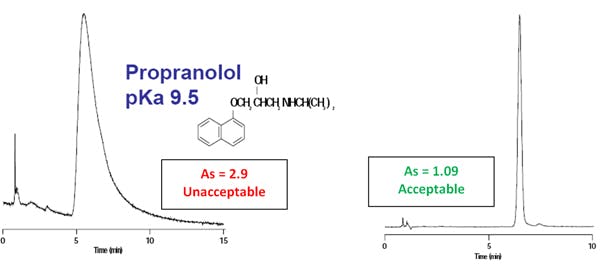
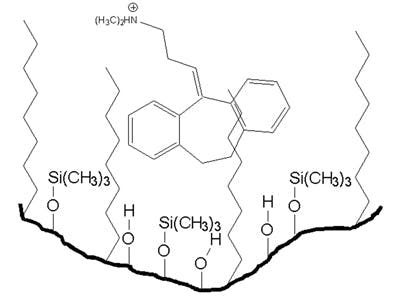
The primary cause of peak tailing is the occurrence of more than one mechanism of analyte retention. In reversed-phase separations, analyte retention is usually achieved through nonspecific hydrophobic interactions with the stationary phase. However, polar interactions with any ionised residual silanol groups residing on the silica support surface are also common. Compounds possessing amine and other basic functional groups interact strongly with such ionised silanol groups, producing tailing peaks. This is illustrated in the figure shown below at a mobile phase pH >3.0.
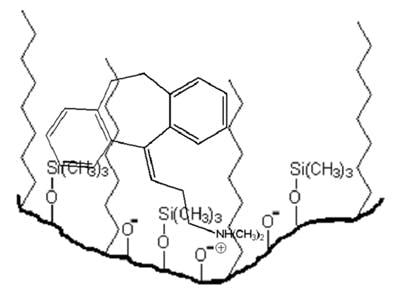
Secondary analyte interactions, with ionised silanols on the silica surface, give rise to peak tailing. These interactions need to be minimised to achieve acceptable peak shapes.
How to avoid peak tailing
There are a few methods that can be used to avoid peak tailing:
If you need assistance choosing the right column, then our HPLC column selection guide will be of help.
Operate at a lower pH
As silanol groups are acidic, secondary interactions can be minimised by performing the chromatographic separation at a lower pH — thereby ensuring the full protonation of such ionisable residual silanol groups.
Of course, this may lead to a decrease in retention time is ionisable basic analytes are present in the sample - which may then require mitigation through a reduction in the organic modifier content of the mobile phase. Standard silica should not be used under pH 3 as this may induce silica dissolution. Agilent ZORBAX Stable Bond (SB) columns are designed to operate at low pH (<3).
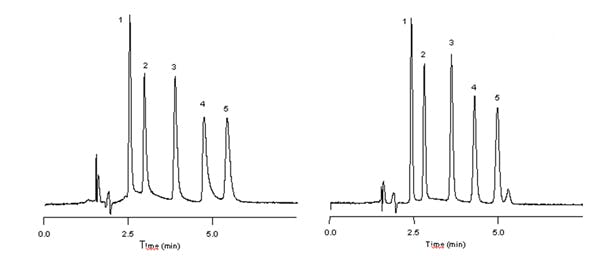
The five component mix of basic drug compounds in the chromatograms below consist of phenylpropanolamine, ephedrine, amphetamine, methamphetamine and phentermine (peaks 1-5) respectively.
The first chromatogram was obtained at a mobile phase pH of 7.0. Under these separation conditions, peak 4, methamphetamine, shows an As of 2.35. Lowering the mobile phase pH reduces basic analyte interaction with residual silanol groups — thereby eliminating excessive peak tailing. The second chromatographic trace was acquired with a mobile phase pH of 3.0, reducing the As of methamphetamine under these separation conditions to 1.33.
Use a highly deactivated column
Alternatively, an ‘end capped column’ can be utilised. End-capping is a process whereby residual silanol groups are treated to convert them to substantially less polar surface functional groups. This has the effect of reducing the amount of secondary interaction they can potentially have with polar analyte molecules.
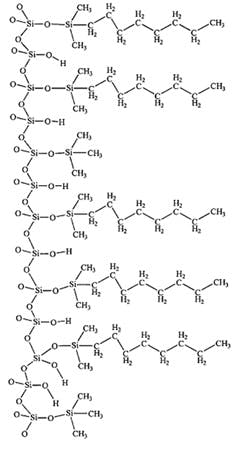
Chemical reagents typically used in the end-capping process include;
- Trimethylchlorosilane (TMCS)
- Hexamethyldisilazane (HMDS)
End-capping reduces the peak tailing seen for polar analyte compounds by effectively blocking their interaction with potentially ionisable residual silanol groups. However, end-capping only reduces further by approximately 50% of the number of unreacted residual silanol groups. Due to steric hindrance factors, such reactions are rarely very efficient in absolute terms, and consequently a “fully end-capped” column is not quite as it may seem!
Agilent ZORBAX Eclipse Plus columns are highly end-capped, deactivated, and are an excellent choice for method development or general laboratory use. They give rise to symmetrical peak shapes even with acidic, basic, and highly polar analytes
Consider the possibility of mass overload
If all chromatographic peaks tail, then consider the possibility that the column has been mass overloaded. Assess this by simply diluting the sample x10 and re-assess the peak shapes in the resulting chromatogram. If necessary, use a higher capacity stationary phase (i.e. increased % carbon or pore size), use a column with an increased diameter, or decrease the absolute sample amount or volume injected
Consider the possibility of column bed deformation
Evaluate the column to ascertain whether the development of a column void or a partially blocked inlet frit are the root cause(s). Substituting the column will quickly confirm the problem. If a void is suspected, reverse the column, disconnect it from the detector, and wash in 100% strong solvent (at least 10 column volumes). This will remove any blocking contamination from the column inlet filter directly to waste. Check the manufacturer’s instructions to understand whether the column should remain in the reversed flow orientation. The blockage of column frits can be avoided though the regular replacement of solvents filers, use of in-line filters, and guard columns. More information on these parts can be found using the links below.
- Solvent & In-line filters
- Guard columns
Work at high pH when analyzing basic compounds
From a practical point of view, it is usually only possible to use pH to suppress acid ionisation, as base ionisation typically requires pH values of greater than 8, at which pH silica dissolution can occur. However, Agilent ZORBAX Extend HPLC Columns are able to operate extended pH, the silica surface being protected from dissolution by the use of bi- and tridentate ligands. These ligands are bidentate and are bridged using proprietary chemistry, prior to their application to the stationary phase. Their bridged structure affords steric protection against hydrolysis of the silica surface.
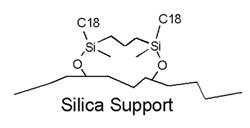
Bidentate Ligands of Agilent ZORBAX Extend HPLC Columns
The possible occurrence of an interfering compound is the primary reason why peak tailing should not be ignored. Confirm the presence of an interferent by changing the detection wavelength, or improve the resolution of the separation by using a column offering greater efficiency i.e. a longer column or a column packed with smaller sized particles. Agilent ZORBAX RRHT and RRHD columns are designed to afford very high efficiency separations which may serve to separate the co-eluting peaks.
Use a sample clean-up procedure
Solid Phase Extraction (SPE) can be used to remove any interfering contaminants.
And... no more peak tailing
Using one, or all of these methods, can put an end to the chromatogram woes brought about by dreaded peak tailing. As with many things in our paradigm of chromatography, the answer is closer than it may seem.
If you want more info on HPLC column selection, check out our useful column selection resource.