18 Feb 2022
MythBusters: I cannot use buffers on my UHPLC system
By Josep M. Serret
Following my last MythBuster about HPLC columns drying up when mobile phase runs out, I wanted to continue on in the theme of liquid chromatography, this time with a commonly encountered UHPLC myth.
So, although chromatographers are generally sensible types, some aren't using buffers in their UHPLC systems. Why? Let's get to the bottom of this one.
I cannot use buffers on my UHPLC system.

I’ve told this story many times: I saw once an HP 1050 whose pump head was completely encrusted in phosphate precipitate, no doubt the result of countless leaks. Spectacular though it was, the system owners were completely unaware because they had never opened the pump cover and looked inside. No need: they hadn’t noticed any change in performance!
Treating a modern UHPLC like this seems utterly unthinkable: everybody knows that UHPLC systems are very delicate, they are just not as robust as our good ol’ HP 1050 (oops, Freudian slip there?) So, it would make perfect sense to avoid buffers in UHPLC altogether. I mean, people doing things like the following chromatograms are just playing with fire:

Figure 1: HIC of protein standard mix. Mobile phase A: 2M ammonium sulphate in 50mM phosphate buffer, pH 7. Mobile phase B: 50mM phosphate buffer, pH 7. Column: Protein-Pak HiRes HIC 2.5um, 4.6x100mm. Column temperature: 30°C. UHPLC System: Waters Acquity Arc Bio UPLC. Adapted from Reference 4.
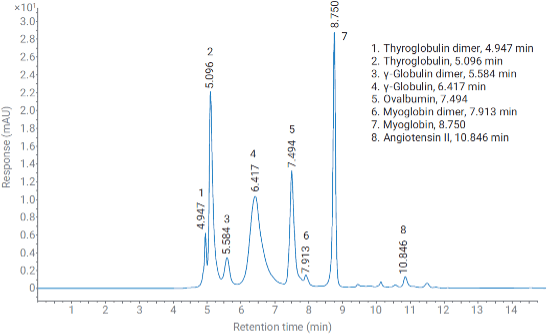
Figure 2: SEC of protein standard mix. Mobile phase: 137mM NaCl and 2.7mM KCl in 10mM phosphate buffer, pH 7.4. Column: AdvanceBio SEC 200A 1.9um, 4.6x300mm. Column temperature: 40°C. UHPLC System: Agilent 1290 Infinity II Bio with ultra-low dispersion kit (0.07mm capillaries). Adapted from Reference 5.
What makes UHPLC systems so delicate? For starters, we push them to extreme pressures, where any failure can have catastrophic consequences. The firmware that controls these instruments is also far more sophisticated than before, with dozens of built-in sensors and system checks that may shut down the system to protect sensitive components. We also expect UHPLC systems to perform better and more consistently than ever before: we demand much tighter tolerances on noise, retention time drift, peak shapes, sensitivity…
Shouldn’t we perhaps demand the same standard of excellence from ourselves? Shouldn’t we be much more scrupulous when we prepare mobile phases and samples for UHPLC? Do we follow the basic rules…
- Use the highest-purity solvents and reagents
- Filter all mobile phases through 0.22µm filter membranes before use
- Inspect eluent reservoir filters, inline filters, and guard columns daily, replace if they look suspicious
- Prepare mobile phases in small volumes, and discard daily
- Avoid leaving solvent bottles on the system for extended periods
- Thoroughly flush system and columns at the end of each batch
The list could go on, but just these few will prevent a host of problems.
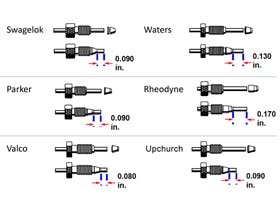
An argument I have sometimes heard is that tubing and fittings in UHPLC systems have such narrower bores that they are much more prone to blockages. A colour coding convention for HPLC tubing internal diameters has existed for many years, with some common specifications being:

A typical analytical HPLC system uses a combination of blue and green tubing to connect different modules, with tubing as wide as twenty “thou” still found in some of the older systems. The first generation of UHPLC systems started to transition to red, which provided lower dwell volumes and improved extra-column dispersion. The latest generation of instruments, and the so-called “low dispersion kits” make extensive use of black capillaries.
Substituting a piece of black capillary for a piece of green tubing of the same length has a dramatic effect on the backpressure. A green capillary has a cross section of approximately:

Whereas the cross section of a black capillary is roughly 6 times smaller:

Consequently, the pressure generated by the black capillary is six times higher, all other factors being equal. But is the black capillary also six times more likely to get blocked?
Let’s follow some of those best practices mentioned above, and filter the mobile phase through a 0.22µm membrane. We can say with confidence that the largest particles in suspension in the eluent are less than 0.22 µm in diameter (see Reference 6). This is around 770 times smaller than the ID of the green capillary, or 320 times smaller than the black. Hardly a recipe for disaster!
There is nothing about a UHPLC system that makes it incompatible with buffers, high salt, or extreme pH. The number one cause of UHPLC breakdown is just poor attention to mobile phase and sample contamination. Consider the simple rules I suggested earlier, follow the recommendations from your user manual, and get the most of these remarkable machines.
Myth number 6: UHPLC systems are incompatible with buffers. BUSTED!
References
1: CHROMacademy, ‘HPLC Solvent Pumping Systems, Lesson 2: Simple Pumping Systems.’ Accessed 14/07/2021.
2: Lee, J. M., ‘A study of porous membrane evaporation for desalination in a flow system.’ Masters Theses, 1968. https://scholarsmine.mst.edu/masters_theses/6935
3: Nelemans, B., Wagemakers, J., ‘Mass transfer inside membranes, explained.’ Aquastill B.V., 2020.
4: Koshel, B. M. et al., ‘Method transfer and routine analysis of protein and peptide-based drug products using a biocompatible UHPLC system.’ Poster presented at Pittcon 2019. Waters Corporation, 2019.
5: Nägele, E., ‘High-resolution SEC with the Agilent 1290 Infinity II Bio LC System.’ Application Note 5994-2709EN. Agilent Technologies, 2020.
6: ‘Defining A Pore Size And Sterile Filtering; 0.2 Micron Vs. 0.22 Micron.’; ‘Nominal vs Absolute: What’s the Difference?’ Sterlitech Inc. Accessed 14/07/2021.