
22 Nov 2021
Green Separation Sciences - Environmental Responsibility
By Josep Serret
As COP26 – the 26th UN Climate Change Conference of the Parties – has been taking place just a few miles from us in Glasgow, we at Element have been reflecting on green chemistry. Through a series of activities, blog articles, and internal training events, we have been taking stock of the impact of chromatography on the environment.
We kicked off with an insightful article full of actionable tips to reduce solvent use and recycle spent consumables. A couple of weeks ago, Crawford and S.C.A.T.™ Europe (Safety Center for Analysis Technology) attracted a lot of attention at Lab Innovations 2021 in Birmingham, where we were showcasing our extensive range of solvent management solutions designed to reduce exposure to harmful solvent vapours and improve the safety of laboratory waste disposal.

Element and S.C.A.T.™ Europe at Lab Innovations 2021. See our LinkedIn post.
Element place sustainability and HSE (health, safety and environmental) concerns at the heart of all their activities. We were recently recognised as a global leader in Environmental, Social and Governance (ESG) policies, and their impressive environmental commitments will see the group becoming “net zero” across all its operations by 2035 (Environmental, Social and Governance | Element).
The environmental footprint of analytical chemistry is well documented: an analysis comprising sample preparation (especially solvent extraction) and liquid chromatography can consume significant amounts of organic solvents, and generate daily several litres of volatile, flammable and toxic waste. Environmental factors have not been high in the chromatographer’s list of priorities: solvents and analytical conditions have been mandated by efficiency, analysis time, method robustness, and running costs. However, method development in the 21st century requires a paradigm shift that places as much emphasis on HSE as on analytical performance.
The Green Chemistry Movement

Green Chemistry aims to “design chemical products and processes to reduce or eliminate the use and generation of hazardous substances” through innovation. It emerged in the early 1990s, in response to the overwhelming impact of industrial chemistry on the environment. Its fundamental tenets were formalised by Paul Anastas and John Warner in their seminal book, “Green Chemistry: Theory and Practice” (1998).
The 12 Principles of Green Chemistry are as follows:
- Prevention
It is better to prevent waste than to treat or clean up waste after it has been created. - Atom Economy
Synthetic methods should be designed to maximize the incorporation of all materials used in the process into the final product. - Less Hazardous Chemical Syntheses
Wherever practicable, synthetic methods should be designed to use and generate substances that possess little or no toxicity to human health and the environment. - Designing Safer Chemicals
Chemical products should be designed to preserve the efficacy of function while reducing toxicity. - Safer Solvents and Auxiliaries
The use of auxiliary substances (e.g., solvents, separation agents, etc.) should be made unnecessary wherever possible and, innocuous when used. - Design for Energy Efficiency
Energy requirements should be recognized for their environmental and economic impacts and should be minimized. Synthetic methods should be conducted at ambient temperature and pressure. - Use of Renewable Feedstocks
A raw material or feedstock should be renewable rather than depleting whenever technically and economically practicable. - Reduce Derivatives
Unnecessary derivatization (use of blocking groups, protection/deprotection, temporary modification of physical/chemical processes) should be minimized or avoided if possible because such steps require additional reagents and can generate waste. - Catalysis
Catalytic reagents (as selective as possible) are superior to stoichiometric reagents. - Design for Degradation
Chemical products should be designed so that at the end of their function they break down into innocuous degradation products and do not persist in the environment. - Real-time Analysis for Pollution Prevention
Analytical methodologies need to be further developed to allow for real-time, in-process monitoring and control prior to the formation of hazardous substances. - Inherently Safer Chemistry for Accident Prevention
Substances and the form of a substance used in a chemical process should be chosen to minimize the potential for chemical accidents, including releases, explosions, and fires.
Although the Principles were written with organic chemistry in mind, many of them can be applied to analytical chemistry.
This is, however, no easy task: as chromatographers, we tend to repeat what has worked in the past. Hence, the majority of RP methods are based on ACN – a toxic solvent derived from fossil fuels – and 4.6x250mm ODS columns, which consume large volumes of mobile phase. Regulated laboratories in pharmaceutical and food analysis also rely on validated legacy methods, which are notoriously difficult to change. But green chromatography is the future, and there have been encouraging innovations in recent years. See the Reference section to learn more.
Reduce, Replace and Recycle Harmful Solvents

We need to reduce our dependency on harmful mineral solvents, and the amount of waste generated by our labs. The three Rs (Reduce, Replace, Recycle) are often mentioned in relation to green analytical chemistry:
- Reduce the use of flammable, volatile, toxic, or expensive solvents
- Replace by shifting to alternative GRAS (Generally Recognised as Safe) solvents
- Recycle, especially in large scale preparative separation technologies
In our time of rapid innovation, it is also easy to explore emerging technologies and alternative separation or spectrometric techniques, with greener credentials than HPLC. While we may not be able to make all our existing methods greener, these overarching principles should always be considered when developing future methodologies.
Reduction Measures
During the global shortage of acetonitrile in late 2008, Ron Majors wrote an excellent article for LCGC North America (see References) discussing simple measures to reduce this solvent in HPLC. The first of Ron’s suggestions was to change column length, internal diameter, particle size or, indeed, all of them. Reducing these parameters results in shorter analysis times (length and particle size) and lower flow rates (internal diameter), all of which reduce solvent consumption.
Significant changes in column geometry can place important demands on a traditional HPLC system: lower dwell volume, optimised extra-column volume (injection system, tubing, and detector flow cell), higher back pressures. More than a decade after Majors wrote his article, with UHPLC systems being ubiquitous, none of these problems should stop us from using smaller, more eco-friendly columns.
We often say at Crawford that there is life beyond C18. Changing ODS for a less retentive stationary phase – such as C8, phenyl, PFP, or C4 – can significantly reduce the amount of organic eluent and achieve similar resolution – or better, through orthogonal interactions – than the original column. Mobile phase pH, ionic strength, and temperatures above or below our trustworthy Tamb (why do we have a column oven again?) are parameters that should be systematically investigated and optimised.
Alternative Techniques
Sample Preparation
The best sample preparation would be no sample preparation, but this is often not possible. Sample extraction and purification is critical, and often the most time consuming and resource-intensive phase of chromatographic analysis. It can also have, regrettably, the biggest HSE footprint.
Fortunately, there have been in recent years great improvements in automation and miniaturisation of extraction methods. Techniques like DLLME (Dispersive Liquid-Liquid Microextraction) and Micro-SPE require just a few microlitres of organic solvent. SPME (Solid Phase Micro-Extraction) and SBSE (Stir-Bar Sorptive Extraction) are truly solvent-free techniques. All of them can be automated for optimal efficiency and minimal exposure to harmful solvents.
Where miniaturisation is not an option, techniques such as Microwave-Assisted Extraction, Ultrasound-Assisted Extraction, or Accelerated Solvent Extraction can process large sample sizes with less organic solvent, and in some cases with water alone.
Liquid Chromatography
It is fair to admit that HPLC, at least in its traditional form, is not an eco-friendly technique. Reversed phase with a binary water/ACN or buffer/ACN mobile phase, using relatively long columns with 4.6mm ID, remains the most common configuration. These systems will typically produce anywhere between 500mL and 3L of waste solvent, every day.
In isocratic mode, some of this solvent can be recycled by an automated switching valve that diverts the solvent back to the reservoir when no peaks are detected. For a typical chromatogram, this can amount to substantial savings.
In gradient mode, straightforward recycling is not an option. In this case, waste solvents are typically collected, segregated in halogenated and non-halogenated drums, and disposed of. Solvent distillation units for on-site recycling are available, but not widespread. In most cases, the fate of the solvents will be determined by the waste disposal company and the commercial viability of repurposing them.
UHPLC are an eco-friendly alternative to traditional HPLC. This accolade, however, depends as much on the method as it does on the system: a good many UHPLC systems out there are not used as UHPLC systems, but merely as high-tech HPLC substitutes! Wherever possible, we should take advantage of these machines and downsize our solvent and analysis time requirements.
Capillary LC and Nano LC, utilising columns with internal diameters as low as 50μm and operating at flow rates in the region of nanolitres per minute, are the ultimate eco-friendly liquid chromatographs.
Gas Chromatography
In terms of solvent economy, GC has the upper hand. With no liquid mobile phase, organic solvents are only required at the sample preparation stage. And, although we often use toxic solvents to inject the sample into the GC (short alkenes, aromatics, and chlorinated solvents are not unusual) the volumes are orders of magnitude smaller than those consumed in mobile phases for LC.
The most objectionable aspects of GC are the high electricity requirements (especially to heat up and cool down the column oven) and the large volumes of scarce and expensive helium used as carrier gas. However, dramatic improvements have also been made in column heating technologies and, especially, gas generators. Benchtop gas generators provide high purity nitrogen or hydrogen as carrier gases, sustainably produced on demand from compressed air and deionised water, respectively.
Supercritical Fluid Chromatography
Only volatile and semi-volatile species can be analysed by GC, which severely limits its applicability. It is possible to derivatise a great variety of compounds to make them amenable to GC analysis, but this approach goes against the 12 Principles. Fortunately, there is an alternative analytical technique, somewhere between LC and GC.
For years a truly dark art, only accessible to the initiated few, SFC (Supercritical Fluid Chromatography) has seen multiple technological innovations and has become a mainstream “green” analytical technique, particularly in chiral separations (Chiral SFC) and preparative chromatography.
Modern SFC instruments and columns are very similar – if not identical, conventional HPLC systems can be converted to SFC systems – to traditional HPLC. The mobile phase, however, is very different: a supercritical gas, typically carbon dioxide. Heated above a critical temperature (31.1°C) and compressed above a critical pressure (73.8 bar), CO2 transitions into a state where its physical properties are between those of a gas (low viscosity) and a liquid (density, hydrophobicity). Low percentages of organic modifiers, for example, 5 to 35% methanol, are added to supercritical CO2 to modulate its polarity and improve sample solubility.
Due to its low viscosity, mass transfer kinetics are improved, and SFC performance is advantageous over comparable HPLC conditions. In preparative chromatography, the greatest benefit is that CO2 evaporates spontaneously at the collection vessel, thus reducing the need to evaporate vast amounts of organic solvents to recover the purified molecules.
Industrial production, compression and distribution of CO2 requires significant amounts of energy, but SFC is nevertheless considered green thanks to the benefits described above, and to the low toxicity of CO2.
Alternative Solvents
Altogether eliminating the need for hazardous fossil solvents is the most significant and long-lasting improvement we can make to our labs. Unfortunately, it is also the hardest. The 2008 acetonitrile shortage provided the perfect opportunity to move away from this solvent, and many of us explored more or less successful alternatives (at least for a while) but eventually, we returned to our old ways when the price of ACN stabilised again.
It may not be possible to replace all hazardous organics with more benign solvents, but significant strides have been taken to that effect. In 2006, Pfizer appointed Dr Peter Dunn as Global Head of Green Chemistry, with the aim of implementing green chemistry across all operations. As part of the new initiatives, solvents were classified in terms of their properties, including greenness, and researchers were given the opportunity to use greener alternatives where possible:
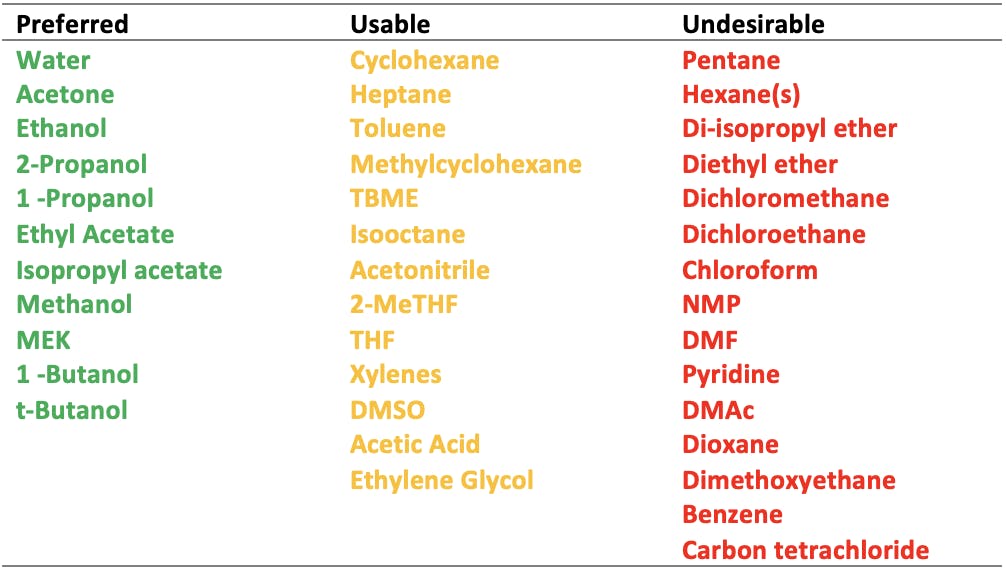
Table 1: Pfizer solvent selection guide, see References

Table 2: Pfizer solvent replacement table, see References
In four years, the use of DCM was halved, and diisopropyl ether was reduced by 99%:

Figure 1: DCM reduction at Pfizer. Source: see References.

Figure 2: Diisopropyl ether reduction at Pfizer. Source: see References.
Some of the more promising and extensively studied alternative solvents for HPLC are listed below:
Water
Water is at the top of every green solvent classification as it is non-toxic, non-flammable, environmentally friendly, and readily available. It has a very low cut-off wavelength, making it a perfect solvent for UV detection, and it is compatible with MS detection. It can also be used with a wide variety of buffers and ionic modifiers.
Applications of totally aqueous mobile phases are common, albeit limited to polar analytes and requiring the use of HPLC columns resistant to dewetting, or phase collapse.
Water has very low elution strength at room temperature, but that changes at elevated temperatures: at 150°C, for example, water has an elution strength similar to that of MeOH/water (50:50) at room temperature. Superheated water chromatography (SHWC) is a chromatographic technique developed around this property.
SHWC requires some specialised equipment: pre- and post-column heaters, and a column oven capable of reaching those high temperatures. Column stability is also a concern since derivatised silica particles are not stable at high temperatures. Common packing materials in SHWC include polymeric phases and organic-coated zirconia. On-column degradation of the analytes must also be considered, as thermally labile compounds will not be suitable for the technique. Despite the limitations, several pharmaceutical SHWC applications have been published.
Ethanol
Hansen Solubility Parameters for acetonitrile and ethanol (see References for source):

Ethanol is less toxic and has a lower vapour pressure than methanol and acetonitrile. It is widely available and less expensive to purchase and to dispose of, making it a good green organic solvent.
It is fully miscible with water and has similar properties (dipole moment, H-donor/acceptor) to MeOH:

It has a higher eluotropic strength than MeOH, so lower proportions of EtOH are needed as modifiers, and several successful substitutions of EtOH for ACN and MeOH have been demonstrated.
However, ethanol has some adverse properties that limit its applicability: in the first place, water/ethanol mixtures are more viscous than mixtures with the other two solvents, leading to increased back pressures. To reduce viscosity, EtOH/water mobile phases are usually used at high temperatures, if care is taken regarding column and analyte stability. Secondly, it has a relatively high cut-off wavelength (210nm), which impairs UV sensitivity and leads to drifting baselines in gradient separations.
Acetone

Acetone has low toxicity and is biodegradable. It has similar physicochemical properties to acetonitrile, including low viscosity and water miscibility.
Unfortunately, acetone has a high cut-off wavelength (approx. 340nm), which severely limits its usefulness with UV detection. It is also very volatile and can cause HPLC pumps to cavitate, resulting in noisy baselines. However, this same property makes it an excellent solvent for evaporative detection, such as ELSD, CAD or MS.
Propylene Carbonate

Propylene carbonate (4-methyl-1,3-dioxolane-2-one, or PC) is an ester derived from propylene glycol, used for cleaning, degreasing, and paint stripping among other applications. It has high boiling and flashpoints, low toxicity and bioaccumulation, and is easy to dispose of as it is biodegradable.
Several applications of PC as an HPLC solvent for bioanalysis and pharmaceutical formulations have been published. Although the results prove that PC is a viable green solvent, they also show two main drawbacks. The first is its limited solubility in water. To improve miscibility, a third solvent, usually methanol or ethanol, is added to “bridge” the two.
The second limitation comes from the high viscosity of PG (2.76cP), which results in high backpressures. The viscosity of PG/ethanol/water mixtures is even greater than that of the pure liquid. Because of this, mass transfer kinetics are slow compared to water/ACN systems, requiring lower flow rates and longer analysis times. Due to the complexity of ternary systems, the design of experiments (DoE) is often recommended from method development.
Ethyl Lactate

Ethyl lactate is non-toxic, biodegradable, easily produced at industrial scale, and fully miscible with water and other organic solvents. Despite its affordability, however, it is difficult to find in HPLC grade.
Like acetone and ethyl acetate, it has a high cut-off wavelength. The biggest disadvantage of ethyl lactate is that it dissociates into ethanol and lactic acid under acidic and basic conditions, limiting its useable pH range.
Ethyl Acetate

Ethyl acetate is considered a green solvent for its low toxicity and biodegradability. It is, however, volatile, flammable, and causes eye irritation. As an HPLC solvent, its usage is limited by low miscibility with water and a high cut-off wavelength. Despite these limitations, isocratic methods have been successfully developed for pharmaceutical applications.
Other solvent systems that have attracted attention include ionic liquids (IL), and water-based eluents containing a small proportion of organic and surfactants – the most common being SDS (sodium dodecyl sulphate) – above their critical micellar concentration (CMC). These mobile phases, combined with reversed phase-type stationary phases, have become known as micellar liquid chromatography (MLC). They have complex and interesting chromatographic properties and are regarded as green because they are non-volatile, non-flammable, non-toxic, and readily biodegradable.
We acknowledge the important impact that analytical sciences have on our environment, and we are always researching new products, protocols, and technologies to reduce the footprint of our labs. We are also committed to helping our customers achieve their own environmental goals. Talk to Element to learn how we can support your transition to greener chromatography.
References
Anastas, P. T.; Warner, J. C., Green Chemistry: Theory and Practice, Oxford University Press, NY, 1998
American Chemical Society, Green Chemistry Institute, https://www.acs.org/content/acs/en/greenchemistry.html
Royal Society of Chemistry, Green Chemistry, Chem. Soc. Rev., 41 (2012) 1405-1608
Majors, R. E., The Continuing Acetonitrile Shortage: How to Combat it or Live with It, LCGC North America, 27, Issue 6 (2009) 458-471
Welch, C. J., et al., Greening Analytical Chromatography, Trends in Anal. Chem., Vol 29, 7 (2010) 667-680
Pfizer’s Green Journey, Greener Processes. https://www.pfizer.com/purpose/workplace-responsibility/green-journey/greener-processes
Yabré, M. et al., Greening Reversed-Phase Liquid Chromatography Methods Using Alternative Solvents for Pharmaceutical Analysis, Molecules 2018, 23, 1065-1090
Hansen Solubility Parameters, https://www.hansen-solubility.com/, accessed 10/11/2021






