25 Mar 2020
Normal Phase flash chromatography
Aim for a low Rf value in TLC, play with your solvents to put yourself in the best possible situation to increase resolution and loading capacity for flash purifications.
A little post out of the usual High-Performance Liquid Chromatography scope this week. I recently had a chat with my colleague Emma who’s done A LOT of flash purifications in her past life and I got all inspired by her lab stories. I’d like to draw your attention to flash chromatography method development and cartridge selection. Probably a quick refresher for most of you, but I hope this can save some of you a bit of money at the end of the day!
Organic chemists often rely on Thin Layer Chromatography (TLC) to get a quick idea of the eluent mixtures they will use for their flash purification on low-pressure instruments. Although reversed-phase (RP) solvent systems are increasingly popular for the separation of polar analytes, normal phase (NP) is still the most common purification mode because NP fractions are a lot easier to evaporate than their water-rich RP counterparts. Because NP separations rely mostly on adsorption rather than partition, NP silica-based flash cartridges are usually considered as consumables. They are changed for every single purification in order to limit carry-overs and fraction cross-contaminations. It is better to know how to select the most appropriate cartridge in order to maintain spiraling consumable costs under control. Let’s see how this can be done.
Loading capacity – the 1% rule still holds…
Loading capacity and sorbent mass are proportional. I think I have always heard of the 1% rule when it comes to the loading capacity of silica-based materials. Who hasn’t? The good thing is that it generally works! For flash cartridges and Solid-Phase Extraction (SPE) too, when you come to think of it. But 1% is also the lower limit of the loading range, as we can see on the below SiliaSep™ loading chart. Depending on the quality of the initial separation (analysts speak of resolution), the loading capacity will roughly vary from 1 to 10% for NP cartridges. And for any given amount to purify, it is easy not to fully use the capacity of our cartridges. For instance, 2g can be loaded on a 25g cartridge or an 120g cartridge – but not at the same cost!
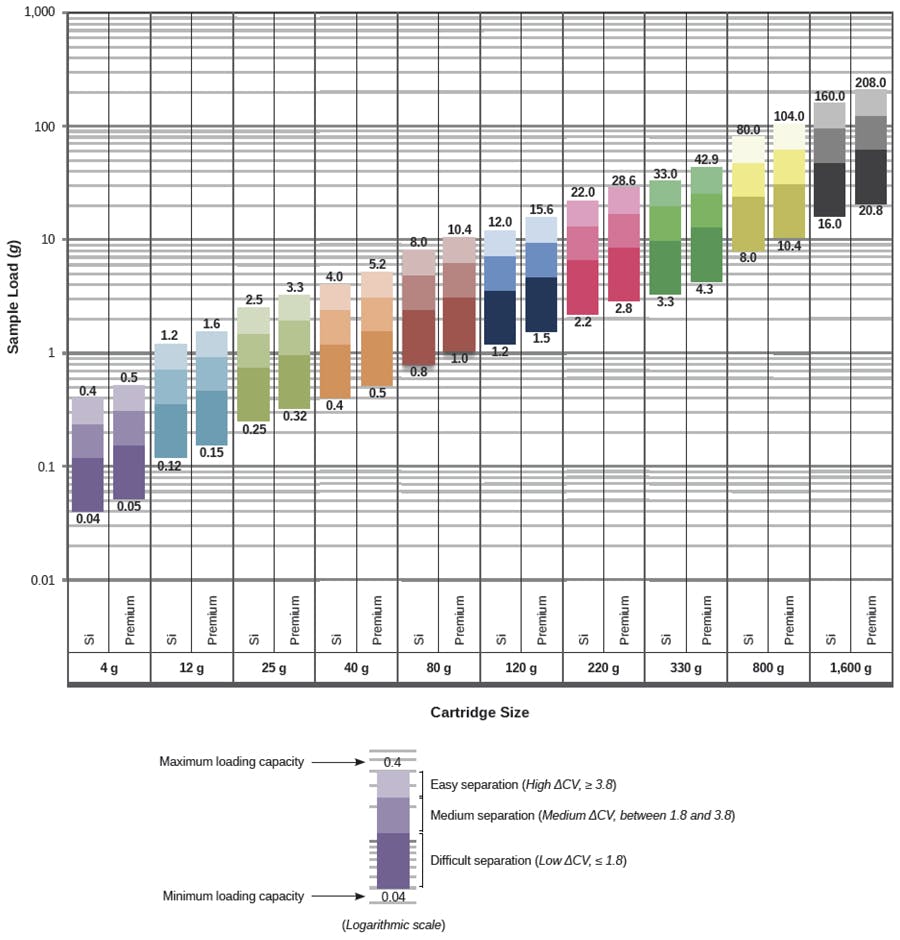
Fig.1 – At least 1%: a loading chart for silica normal phase cartridges.
Smaller spherical particles for high efficiencies?
On this chart, it seems that slightly higher masses can be loaded on Premium cartridges. So why can we load more on these carts when considering identical bed masses? What’s the difference between these Si and Premium carts? Their grades and shapes! Si particles are irregular and range from 40 to 63 µm whereas Premium particles are spherical with a narrow particle size distribution centered on 25 µm… Van Deemter demonstrated in 1956 that the quality of particle manufacturing and packing had a dramatic effect on peak broadening. Since then, analytical chemists have been well aware of particle shapes and diameters and how they affect packing homogeneity, peak sharpness and, ultimately, efficiency. Since the recent introduction to the market of flash carts packed with spherical particles ranging from 20 to 25µm, the feedback havs been positive and higher loading capacities have been recorded.
Higher resolution for higher loading capacities
Efficiency for resolution, then! But efficiency is just one of three major parameters affecting resolution. How about retention and selectivity? Back to chromatography fundamentals, with the below figure!
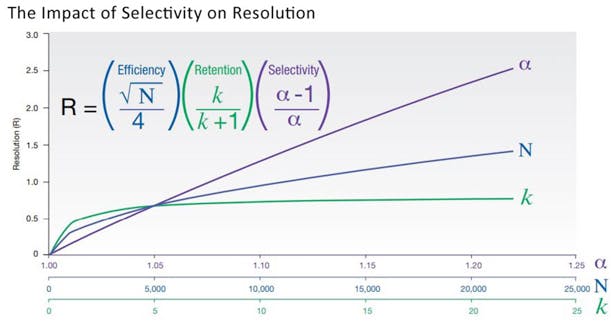
Fig.2 – Not all about efficiency: resolution.
In order to get a good separation, sharp peaks are not enough. Method developers know that a minimal retention is needed in the first place, and selectivity – or the retention difference between analytes – is the most important parameter of all. To optimize these parameters in flash chromatography, chemists usually screen different solvent systems, and more rarely evaluate different stationary phases (bare silica is by far the most popular sorbent).
In flash chromatography, retention is expressed as the number of column volumes (CV) required to elute a component off the column and a measure of selectivity is given by the difference of CVs for two analytes:
ΔCV = CV1 – CV2
But how is this ΔCV transferred to TLC, which is the starting point for most flash purifications? In TLC, retention is expressed as a retention factor (Rf), with low Rf values for analytes that are well retained by the stationary phase and have a poor affinity for the mobile phase. Rf and CV are therefore inversely proportional:
CV = 1 / Rf and ΔCV = CV1 – CV2 = (1 / Rf1) – (1 / Rf2)
Because of this relationship between Rf and CV, good flash separations (or high ΔCVs) are achieved with lower Rfs, the ideal range being between 0.1 and 0.3. In this range, even low ΔRf values are successfully transferred as satisfactory flash separations – and purifications. The below chart presents ΔCVs according to Rf1 and Rf2 values.
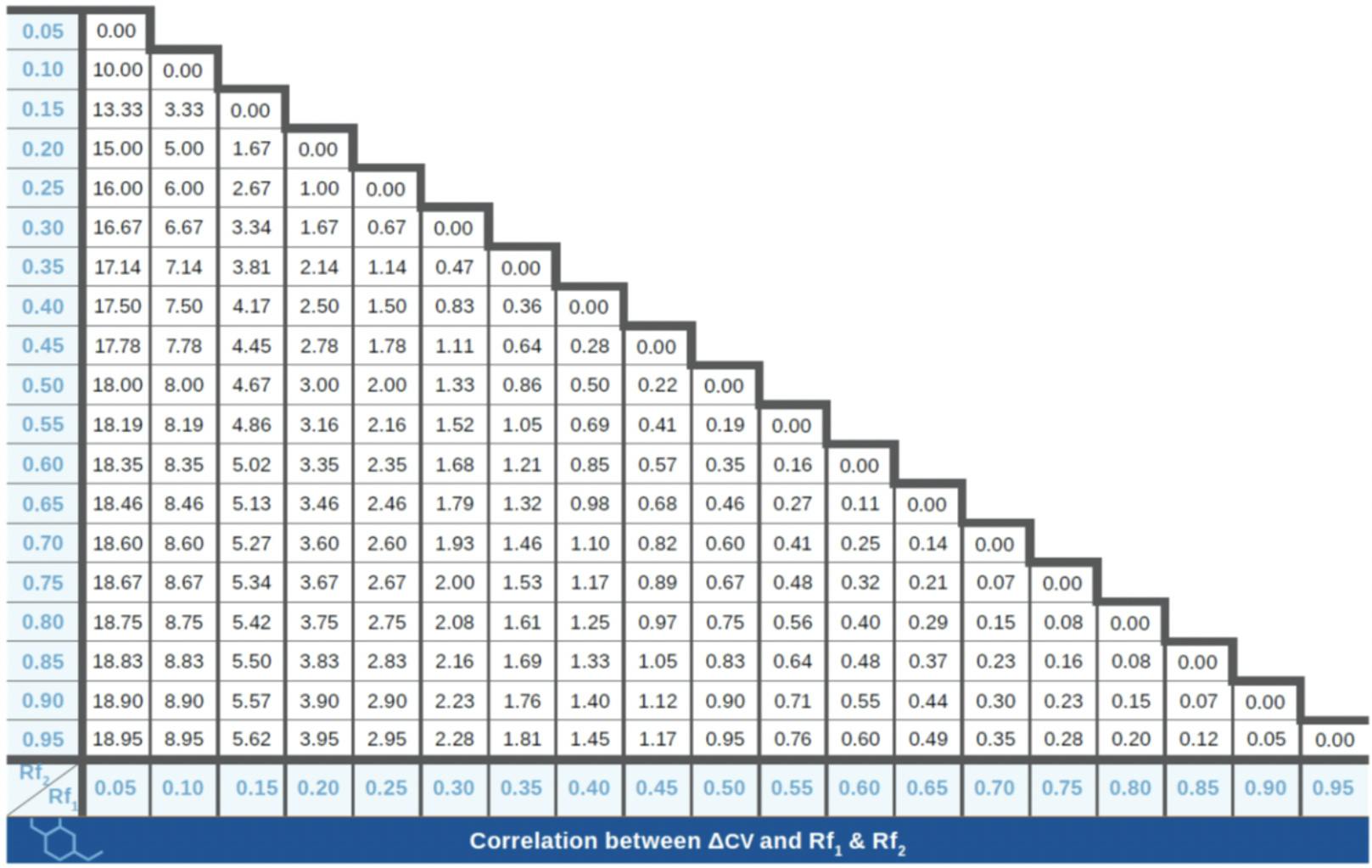
Fig.3 – Resolution in flash chromatography is defined as ΔCVs by Rf1 and Rf2 in TLC.
Towards smaller mixed fractions and higher purification yields
There are several strategies to improve flash separations and, as a result, increase the loading capacity on flash cartridges. Substantial gains in purification yields can be made from method optimization. It is certainly worth the time invested in this extra TLC experiment!
Different solvent systems can be screened in TLC, the most popular being ethyl acetate / hexane and methanol /dichloromethane. The polarity of the analytes and their solubility in the solvents will help select the most appropriate system. In order to increase component migration, increase the relative proportion of strong (or more polar) solvents: ethyl acetate and methanol, respectively. To decrease Rfs, decrease the polarity of the eluent. In order to ensure that ionizable analytes have a good affinity for the solvents and to avoid their adsorption on the silica, it can be useful to add to the mixture 0.1% triethylamine (TEA) or 1% ammonia (easier to evaporate) when working with basic analytes. Again, the optimal solvent system is achieving good ΔRf values with Rfs between 0.1 and 0.3.
While stationary phase screening is rather common in HPLC, it is less common in TLC and silica is the preferred coating. It is probably because almost 80% of separations can be achieved on this sorbent. Now different chemistries are available for both TLC plates and NP flash cartridges (for easy method transfers, we recommend to use the same sorbent in TLC and flash): amino (NH2, for bases and sugars), cyano (CN, less polar than silica, for polar organics), diol (quite popular lately, for saccharides and other polar analytes). I have so far heard of only one purification lab looking at different NP chemistries for screening purposes. Their results were promising. Maybe a new approach to flash chromatography to implement in your own lab?
Take-home message
1) Screen solvent systems and different sorbents in TLC (aim for Rfs between 0.1 and 0.3)
2) The greater the ΔCVs, the more sample loaded on the flash cartridge
3) The greater the ΔCVs, the higher the purification yield
4) Save money by selecting the appropriate cartridge!
Both charts were taken from SiliCycle Purification catalogue.
Remember you can always purchase SiliCycle SiliaPlate™ TLC plates and SiliaSep™ flash cartridges in our shop.