24 Aug 2017
Going back in time - Ternary and Quaternary Gradients
Until recently, I hadn’t heard of ternary or quaternary gradients being used for many years. They have gained a reputation for being somewhat difficult to reproduce – less robust if you like.
However, we shouldn’t let an outdated objection rule out what can be a very useful technique to optimise difficult or complex separations. Modern quaternary HPLC systems are capable of accurately and reproducibly delivering three or four component eluents and make the practical, everyday use of ternary and quaternary separations a reality. It is without doubt that these separations are more complex, but not to the extent that we should not consider their use.
As chromatographers we know how powerful selectivity is in achieving good resolution, and we know how many different column chemistries are available to help achieve good selectivity. Yet mobile phase selectivity is often overlooked as another tool in our analytical armoury which can be both powerful and convenient in developing successful separations, especially for multi-analyte samples.
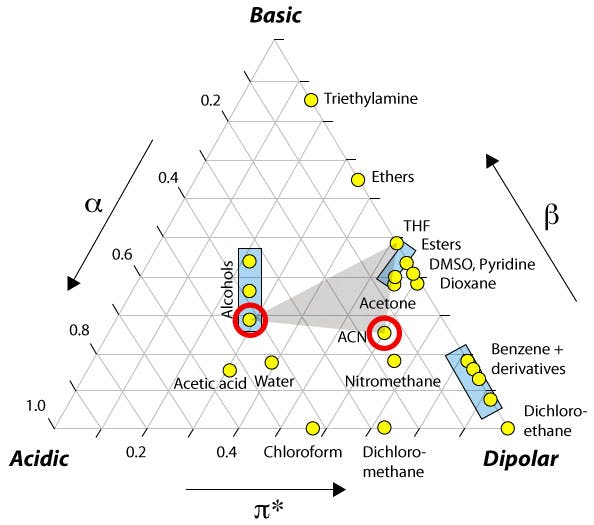
You may have encountered the Snyder solvent selectivity triangle [1] shown in Figure 1, based on the work of Rohrschneider [2], which highlights the acidity (proton donating capability), basicity (proton accepting capability) and dipole characteristic for a range of solvents. The position of methanol and acetonitrile are shown as red circles on the figure which highlights that methanol, as a protic solvent, has stronger proton donating capability, whilst acetonitrile has a stronger dipole interaction characteristic. This goes some way to explaining the relative eluotropic strength of each solvent as well as the differences in selectivity when changing organic modifier from one solvent to the other.
 |
|
| Figure 1: The Snyder solvent selectivity triangle. |
Anyone who has worked with reversed phase HPLC will intuitively know that acetonitrile is slightly more strongly eluting than methanol, especially when lower amounts of organic modifier are used relative to the aqueous component. However it’s the selectivity that we are interested in here and the various mechanisms and interactions involved between mobile phase, stationary phase and analyte that can be somewhat correlated to the Synder Triangle in Figure 1. That is to say – when changing organic modifier, even when the solvent strength is balanced (using a nomogram for example) to obtain an overall similar window of retention, the separation will look different and resolution values between certain peaks will change. This is demonstrated in Figure 2, where I have shown that for a mixture of agrochemical compounds, the optimum organic composition for methanol / water and acetonitrile / water mixtures do not produce a satisfactory separation – both separations show different critical peak pairings. However, when one uses a 1:1 mixture of acetonitrile and methanol as the organic modifier, then a more satisfactory separation can be obtained.
| Column: | Agilent ZORBAX Eclipse Plus C18, 2.1mm × 50 mm, 1.8 µm |
| Mobile Phase: | A = Water (0.1% TFA) |
| B = Acetonitrile | |
| C = Methanol | |
| Flow rate: | 0.4 mL/min |
| DAD: | 270/10 nm, Ref 360/100 nm |
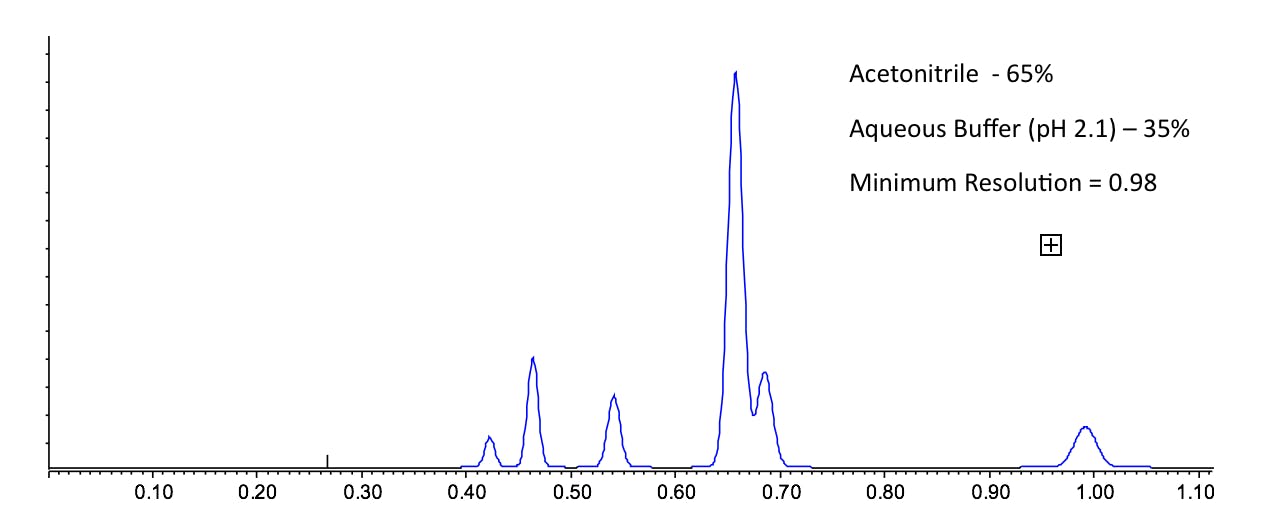
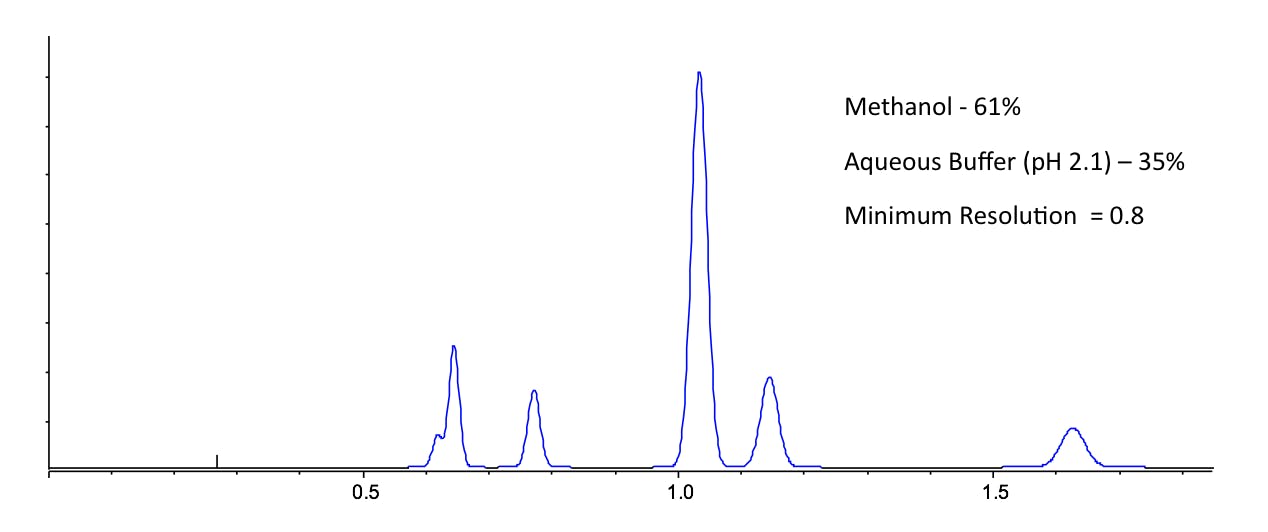
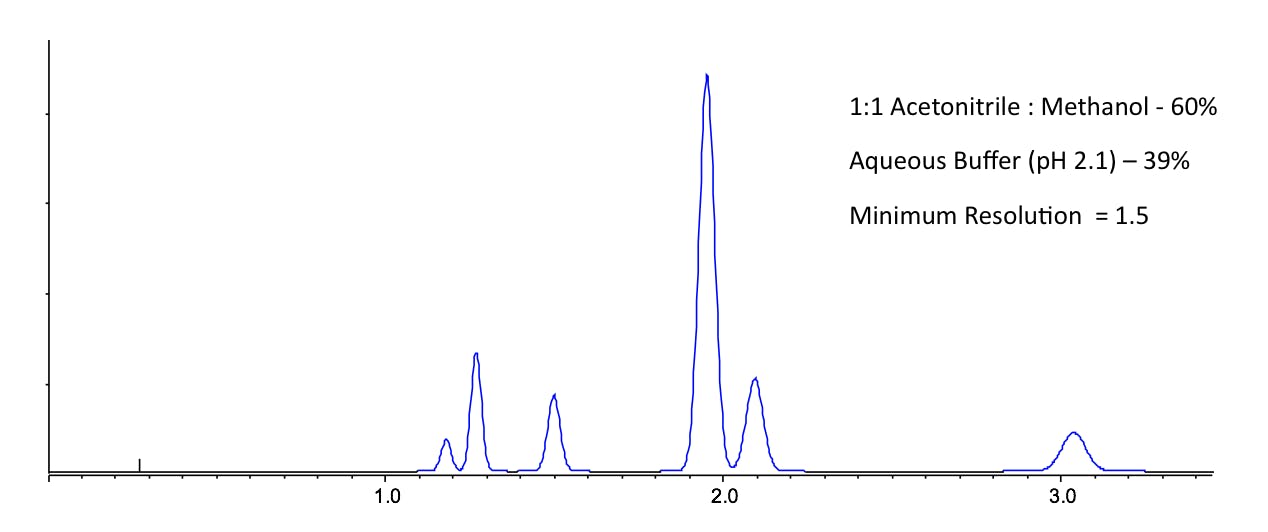
Figure 2: The separation of six environmentally persistent pollutants under the conditions shown to investigate the selectivity effects of changing organic modifier
My general point here is that one might explore the two different solvents for an optimum separation prior to switching to a different column chemistry. This is relatively convenient on a quaternary pump with three solvents lines – methanol, acetonitrile and the buffer component.
We did not explore gradient elution in this case, but the principles remain the same. A gradient separation produced with methanol is capable of producing a different selectivity than one carried out with acetonitrile and both will differ from a gradient separation carried out with a mix of solvents as the organic modifier.
Whilst switching from acetonitrile to methanol (or vice versa) is reasonably straightforward during method development or optimisation, I accept that using blended organic modifiers is much less so. However, one might adopt a fairly generic approach to screen the separation initially and the use of a fourth pumping channel to deliver a constant ionic strength buffer or additive often simplifies the development a great deal.
In the following example, a 1:1 mixture of acetonitrile and methanol is used as the modifier, delivered from separate channels, and 5% of a 2% (v/v) solution of TFA is used to constantly deliver 0.1% TFA during the gradient analysis. The gradient timetable is shown in Figure 3 alongside the separation of Tramadol and its related impurities using methanol, acetonitrile and a 1:1 mixture of each as modifiers. In fact, in stability indicating analysis such as this one, where analyte chemistry and concentration may vary widely, exploring the selectivity effects of various organic modifiers can be an excellent way to optimise these often complex separations and is often far more convenient than screening a wide range of differing column chemistries with subsequent mobile phase optimisation.
| Column: | Agilent ZORBAX Eclipse Plus C18, 2mm × 100 mm, 3.5 µm |
| Mobile Phase: | A = Water |
| B = Acetonitrile | |
| C = Methanol | |
| D = 2% TFA in Water | |
| Gradient ternary ACN: | 0 min - 15 % B, 5% D |
| 8 min - 45% B, 5% D | |
| Gradient ternary MeOH: | 0 min - 15% B, 5% D |
| 8 min - 45 % B, 5% D | |
| Gradient quaternary: | 0 min - 7.5% ACN; 7.5% MeOH, 5% D |
| 8 min - 22.5% ACN, 22.5% MeOH, 5% D | |
| Flow rate: | 1.2 mL/min |
| DAD: | 270/10 nm, Ref 360/100 nm |
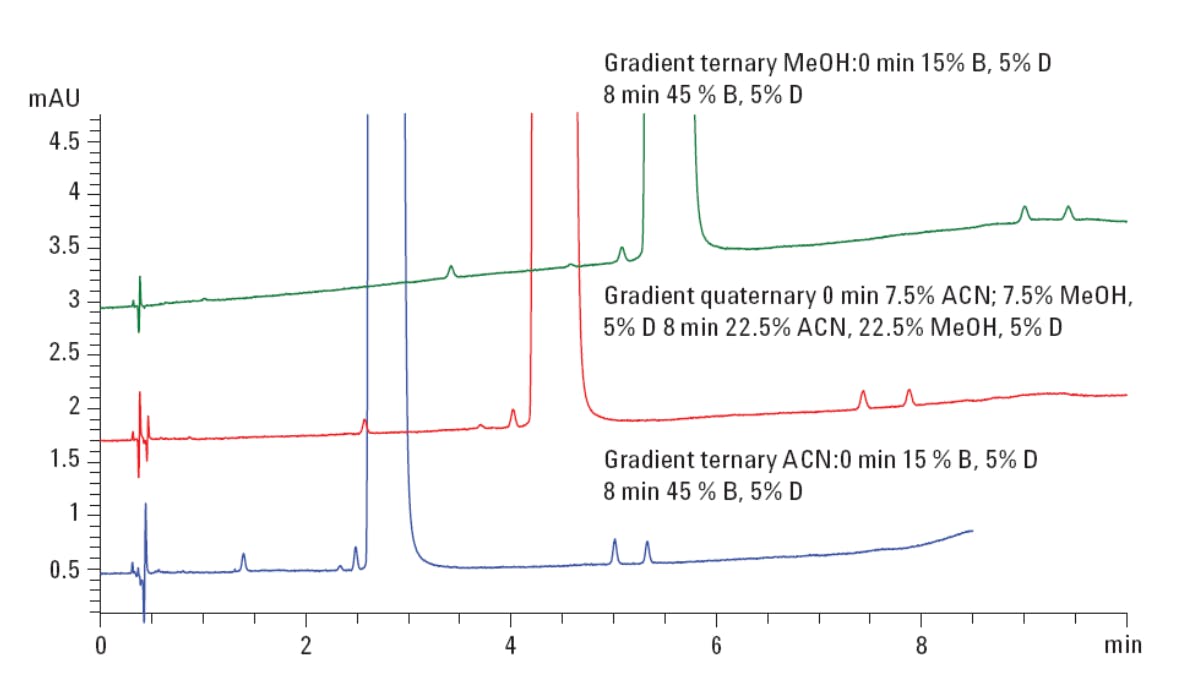
Figure 3: Selectivity optimisation of the separation of tramadol and related impurities.
(Reproduced from Agilent Technologies application note 5990-6439EN).
Using this simple gradient elution of a 1:1 mix of the modifier solvents and a constant ionic strength of the pH modifying TFA, good separation and analysis time have been achieved. More extreme examples might be cited from my own experience, where minor impurities close to the front or trailing edge of the active ingredient peak can be subtly resolved by altering the gradient slope and/or range.
So the next time you have a complex separation to develop, or you really wish you could finesse the separation of an existing separation to make it more straightforward operationally, why not consider the use of ternary or even quaternary eluent systems? The hardware is certainly robust enough to cope – I wonder then why these separations have fallen so badly out of fashion?
(1) L. Snyder, J. Chromatogr. 92, 223 - 230 (1974).
(2) L. Rohrschneider, J. Chromatogr. 22, 2 (1966).