22 Apr 2024
HILIC – The Rising Star of Polar Chromatography
What is HILIC and how does it work?
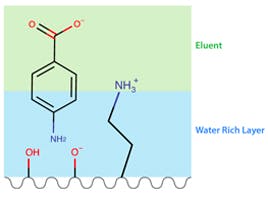
Hydrophilic interaction liquid chromatography (HILIC) is a chromatographic technique which involves a variety of retention mechanisms. HILIC utilises normal phase ‘like’ polar stationary phases (e.g. silica or polar bonded), allowing the stationary phase to be hydrated with a slow-moving layer of water, facilitating the preferential retention of hydrophilic analytes versus their hydrophobic counterparts, via partitioning between water rich and solvent rich phases. HILIC mobile phase compositions are the inverse of reversed phase chromatography, using a combination of aqueous (water/buffers) and organic solvents in which the aqueous component acts as the strong solvent, and is the reason HILIC is often referred to as reverse-reversed phase chromatography [1].
Despite being first proposed in the 1970’s, HILIC has only recently started to gain more traction as a routine technique, probably due to the versatility and relative simplicity of the reversed phase technique and the existence of normal phase chromatography to retain and separate highly polar analytes. Given the increased requirement for polar molecule analysis in the fields of pharmaceutical and biopharmaceutical analysis, and the increasing focus on the environmental impact of solvents used in normal phase chromatography, there has been a requirement to get to know this technique better. HILIC is now being utilized for the separation of a plethora of small and large hydrophilic molecules including carbohydrates, amino acids, glycoproteins, nucleosides, peptides, proteins and many more [1][2]. The increasing demand for HILIC applications has driven much research into the retention mechanisms of the technique and the routine use and method development paradigm of HILIC separations is now better understood.
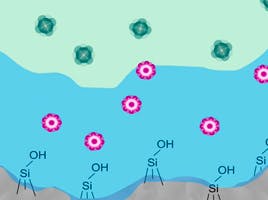
Comparing HILIC to other chromatographic techniques, such as normal phase, would lead us to believe retention and elution are dictated by competitive polar adsorption of either the solvent molecules or the analyte onto the polar stationary phase, through specific polar interactions such as hydrogen bonding. Whereas, for reversed phase retention, the main mechanism of retention is analyte partitioning at the mobile / stationary phase interface. This is where HILIC can become a little more complex than these traditional methods, as the mechanism of retention is a combination of partitioning, adsorption, and electrostatic interactions [3]. Partitioning occurs between the adsorbed aqueous layer associated with the stationary phase and the bulk mobile phase, driven by the analyte’s hydrophobicity [4]. Electrostatic interactions in HILIC occur between charged analytes, charged silanol species of the stationary phase or charged bonded phase ligands. The HILIC stationary phase ligands can undergo ionisation, combining this with different eluent pH’s can be a critical factor in HILIC retention as it can control both the degree of ionisation of the analyte and the stationary phase (silanols and bonded phase) [2].

Figure 1. Diagram representing the possible retention mechanisms involved in HILIC
The complex nature of the retention mechanism makes the elution order of HILIC analysis harder to predict than that of normal phase or reversed phase, thus the importance of screening HILIC stationary phases cannot be underestimated.
The importance of screening …
The rise in demand for HILIC analysis has led to column manufacturers expanding their HILIC column range as there is no single, fully versatile, HILIC stationary phase. Traditional HILIC stationary phases consisted of bare silica, however the variability and acidity of the bare silica surface (due to varying metal ion content and silanol configuration respectively), made it challenging to reliably analyse polar compounds. The increasing popularity of the HILIC mode has led to manufacturers offering a more diverse range of stationary phases to ensure analysts have a choice of phase selectivity at their disposal [5][6]. These, typically, chemically bonded, modified, stationary phases offer superior peak shape and allow the use of mobile phase pH and ionic strength as primary selectivity variables during method development. The wide range of HILIC stationary phases now available include;
- Silica (Type B &C silica preferred)
- Diol and Polyol (Fructan)
- Cyano
- Amino
- Alkylamide
- Amide
- Urea
- Mixed mode (hydrophobic ligands incorporating weak or strong ion exchange moieties or zwitterions)
- Succinimide Derivatives
Some examples of high-quality orthogonal selectivity HILIC stationary phases available from Element Laboratory Solutions include, YMC-Accura Triart Diol HILIC, Agilent Poroshell 120 HILIC-Z (zwitterionic) and AMT HALO Penta-HILIC (polyol).
Stationary phase screening and selection is critical in HILIC analysis to ensure suitable selectivity and the highest peak capacity for the separation under development. The importance of screening is highlighted in the following analysis of vitamins from Agilent Technologies.
Stationary phases:
- HILIC- Traditional bare silica
- HILIC-OH5 - Fructan chemistry with alternative selectivity and excellent peak shape for a wide range of polar compounds
- HILIC-Z - Zwitterionic chemistry with a proprietary bonding technique that offers powerful separation, stability across wide a wide pH range, and excellent peak shape. [6]

Figure 2. Separation of water-soluble vitamins, comparison of Agilent Poroshell 120 HILIC-OH5, HILIC-Z and HILIC columns. Column 2.1 x 100mm 2.7µm. Mobile phase A: 100mM ammonium acetate +0.5% acetic acid (approx. pH4.6) in water. Mobile phase B: acetonitrile. Flow: 0.5mL/min. [6]
From this screen of three different phases, we can see all columns do not successfully separate all analytes, and that peak shape, retention and sensitivity are different across the three phases. This initial column screening can help determine the stationary phase which provides the best starting point for method development. The HILIC-OH5 column shows no co-elution with all peaks well resolved, whereas the HILIC-Z and HILIC column both show co-elution of some peaks, suggesting a more in-depth method development process would be needed as alternative buffers, pH and gradients may need to be investigated to achieve the desired separation. Additionally, the difference between each separation further highlights the unpredictability of HILIC analysis as the elution order of compounds varies on all three phases, demonstrating how the multiple retention mechanisms involved can drastically influence retention.
This further example of screening HILIC stationary phases from Advanced Material Technologies (AMT), demonstrates that HALO Penta-HILIC and HALO HILIC, under the same conditions can lead to different analyte selectivity and retention.

Figure 3. Comparison between HALO HILIC and Penta-HILIC columns [7]
Comparing chromatograms from these phases allows the analyst to evaluate the best starting point for their method development, allowing the most efficient optimisation of eluent and gradient conditions.
HILIC screening is not limited to different stationary phase chemistries, but may also incorporate alternative mobile phase compositions, buffer, and buffer strength as well as eluent pH, all of which can critically influence the retention via the multi-modal separation mechanisms described earlier.
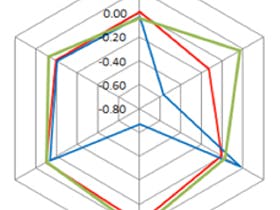
To further highlight this point, a relatively novel use for the HILIC technique is oligonucleotide analysis. Traditionally this analysis is carried out using reverse-phase ion-pairing and ion-exchange chromatography, however in recent years there has been increased focus on the HILIC technique due to the mass spectrometry friendly eluent conditions compared to reversed phase ion pairing and ion exchange and the highly polar nature of oligonucleotides. The influence of eluent pH cannot be underestimated as demonstrated by the separation shown in Figure 4, featuring a YMC Accura-Triart Diol-HILIC column [8].

Figure 4. Influence of mobile phase pH on the separation of an all PO RNA mixture using an acidic (red), a neutral (blue) and basic pH (green). Column: YMC Accura-Triart Diol HILIC (3 μm, 12 nm) 150 x 2.1 mm ID (bioinert coated hardware). [8]
There is minor change with regards to the retention of the internal standard, caffeine, whereas the oligonucleotides show significant retention and resolution differences between the three eluent pH values. Analysis at high pH significantly reduces the retention time of the analysis but limits the resolution between the 20 and 21-mer oligonucleotide, compared to low pH which shows a significant increase in retention and improved analyte resolution. Without screening at neutral pH, the preferred pH might have been pH 9.5, and with extra time spent on development resolution this separation might be improved. However, a comprehensive screen, covering a wide pH range allows an almost ideal separation to be derived without much further method development effort.
As it is difficult to predict the dominant retention mechanism for HILIC analysis the importance of screening multiple stationary phases and pH ranges can be fundamentally important as the initial step in method development.
Element Laboratory Solutions screening toolkit…
The importance of HILIC screening has been highlighted, but with so many HILIC stationary phases entering the market, which ones will work best for your application? At Element we have compiled a comprehensive HILIC screening kit, which involves columns from several manufacturers and different column chemistries to offer a robust, reliable and highly selective screening kit.
The recommended screening kit:
- Agilent Poroshell 120 HILIC-Z column
- YMC-Triart Diol-HILIC
- YMC-Pack NH2 HILIC
- AMT HALO Penta-HILIC
These four columns allow for a wide screen of analytes: polar, acidic, neutral, and basic compounds. Additionally, simultaneously screening columns allows for extra benefits with respect to greener, more sustainable analysis, as a screen saves time, energy, and solvent in the method development process, which otherwise might take many iterative experiments to select the ideal separation conditions.
Our selection of HILIC columns is by no means limited to the four columns stated above, our technical team can work with you and make further recommendations for HILIC columns which will be best suited to your analytes and applications.
Get in touch with an Element Laboratory Solutions sales representative to discuss the best HILIC screening options for your analysis.
References
[1] Chromacademy: HILIC-mechanism-and-stationary-phase
[2] Greco. G and Letzel. T (2013) Main Interactions and Influences of the Chromatographic Parameters in HILIC Separations, Journal of Chromatographic Science, vol 51, pg 684-693, doi:10.1093/chromsci/bmt015
[3] Guo, Y. and Baran, D. (2023) Hydrophilic Partitioning or Surface Adsorption? A Quantitative Assessment of Retention Mechanisms for Hydrophilic Interaction Chromatography (HILIC). Molecules, vol 28, 6459
[4] David V. McCalley (2008) Hydrophilic Interaction Chromatography, LCGC international.
[5] Buszewski B and Noga S. (2012) Hydrophilic interaction liquid chromatography (HILIC)--a powerful separation technique, Anal Bioanal Chem, 402(1):231-47. doi: 10.1007/s00216-011-5308-5.
[6] Agilent Technologies, Hydrophilic Interaction Chromatography Method Development and Troubleshooting, Technical note
[7] Advanced material technology, Halo Catalogue AMT, page 14.
[8] YMC Technical note, Determination of optimum method parameters for the analysis of oligonucleotides via HILIC.