15 Jan 2020
Thinking Outside The Box On Headspace Sampling For Gas Chromatography
Frustrated with static headspace sampling?
Many chromatographers face challenges in which static headspace has been unable to deliver an analytical solution or has taken an inordinate amount of time to optimise.
Solid matrices, polar analytes in polar matrices, volatile matrices, very low analyte concentrations, less volatile analytes and issues around relative response factors (or relative extraction factors)— they’ve all been conspiring against us!
There are the usual approaches: optimising sample to air volume ratio (β), equilibration time and temperature, agitation ferocity (!), salting out*, co-solvent addition** and injection volume (actually injection time with a loop sampler on an instrument). These have all been undertaken during various challenges and whilst some approaches have led to better analytical performance, experimental design approaches are often suited to dealing with the many interactive variables.
This isn’t the worst thing in the world. After all, the job of an analytical chemist involves optimising experiments with inter-related variables and dealing with challenges. However, it seems that if headspace GC methods need to be developed, it is a great deal of effort to get anywhere near decent performance.
Nonetheless, it’s helpful to consider more generic approaches which offer more comprehensive results.
These discussions often lead to the possibilities of dynamic headspace extraction (sampling) with thermal desorption as the method of sample introduction—which can prove somewhat nerve-racking! Dynamic headspace seems even more complicated; extra equipment, different adsorbent tubes to choose from, a whole other set of conditions to optimise for the thermal desorption unit, cryogenics or cold trapping. The options can take their toll on the mind! Or perhaps this scientist is becoming old and grumpy…
However, there are one or two attention-grabbing approaches that offer some hope that there may be more comprehensive techniques available for extraction and the introduction of volatile or semi-volatile analytes. These options can be quantitative (if required) and may not need large scale optimisation. Let's see…..
The Best Options For Headspace Sampling in GC
Dynamic Headspace Sampling
Dynamic headspace sampling (DHS) is achieved using a constant flow of purge gas through the headspace of a conventional headspace vial. These are usually 10 and 20 mL vials, as with static headspace sampling; however, alternative vial sizes are available if required for the optimisation of the sample to headspace ratio (β).
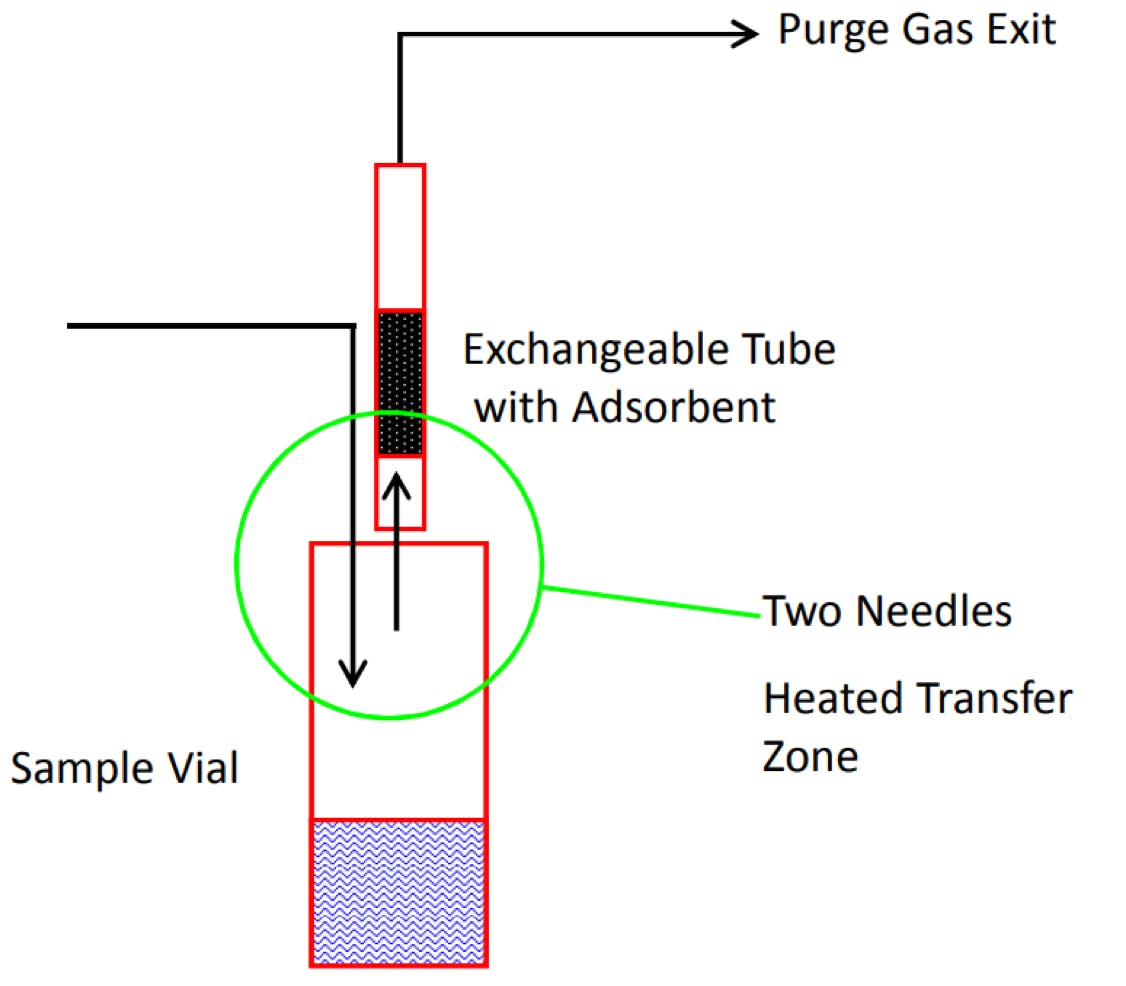
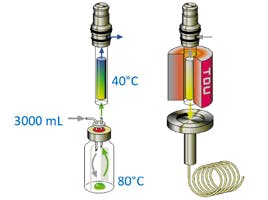
Figure 1: Schematic diagram of a dynamic headspace sampling process
Because the headspace within the vial is constantly purged, the dynamic technique does not rely on a fixed equilibrium within a closed system (i.e. static headspace). As analytes are continually removed from the headspace above the sample, this allows further analyte molecules to be released into the headspace. All analytes are trapped onto an adsorbent prior to liberation in the heated desorption unit. Of course, the sample vial may be heated and agitated as with static headspace sampling.
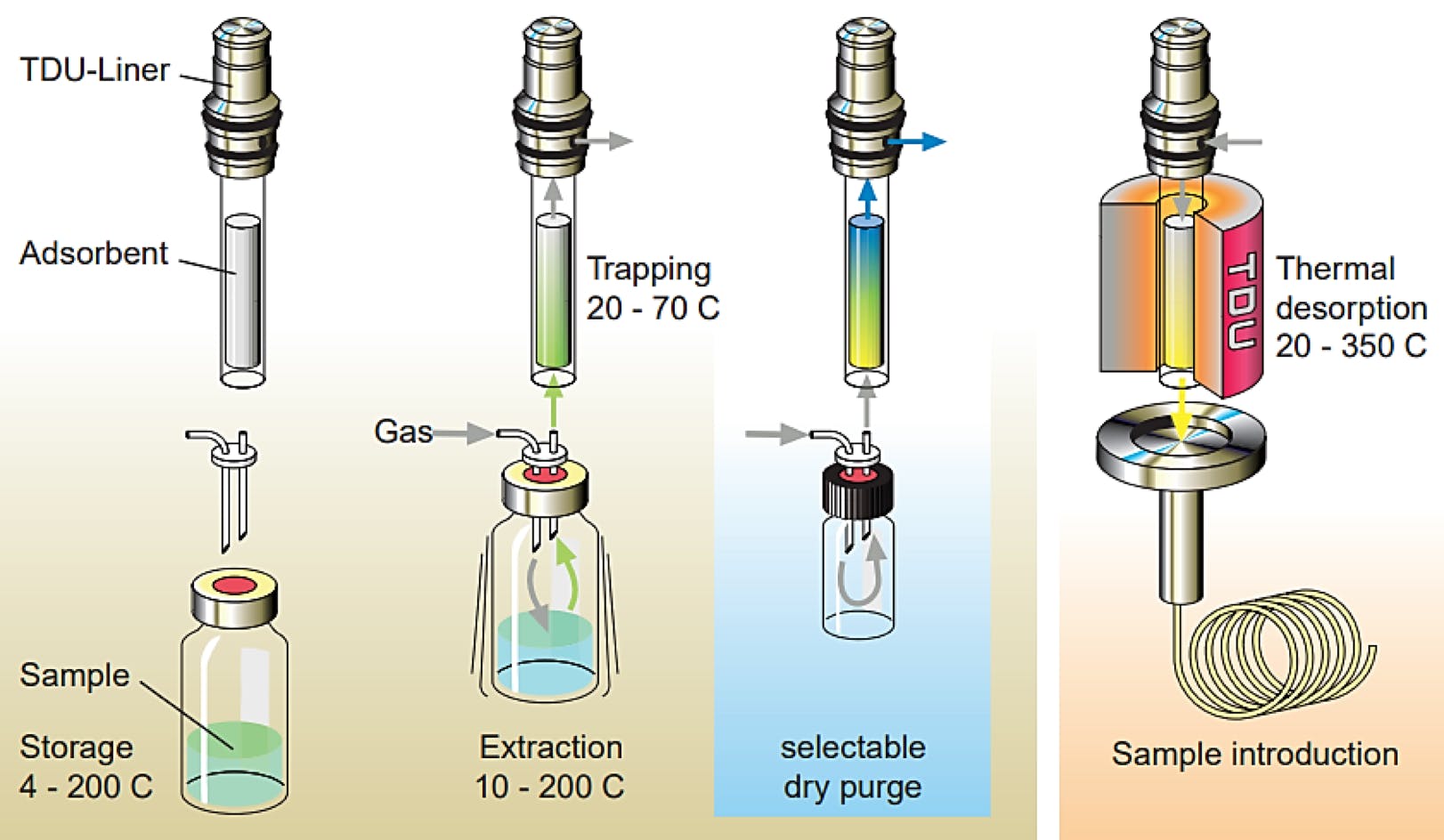
Figure 2: Schematic representation of an automated dynamic headspace extraction (TDU – Thermal desorption unit) (Courtesy of Gerstel GmbH)
Adsorbent selection is an experimental variable; however, adsorbent tubes with multiple packings are available which take some work out of this process (more on this later). The dry purge stage of the process may also require optimisation, although this tends to only be necessary when using aqueous-based matrices. Further, this particular equipment is automated, enabling any experiments to be carried out unattended, even when using a design of experiments approach—meaning working time doesn’t need to be taken up attending to equipment! Indeed, with this system, not only can the experimental variable be altered—but the type of adsorbents used can also be selected. Therefore, the selection of the optimum sorbent will be ready following an unattended overnight campaign.
It should be noted that to achieve higher chromatographic efficiency, it is possible to use cryo or cold-trapping to refocus analytes as they are released from the sorbent trap during thermal desorption. A thermal desorption/cooled inlet combination is shown in Figure 3.
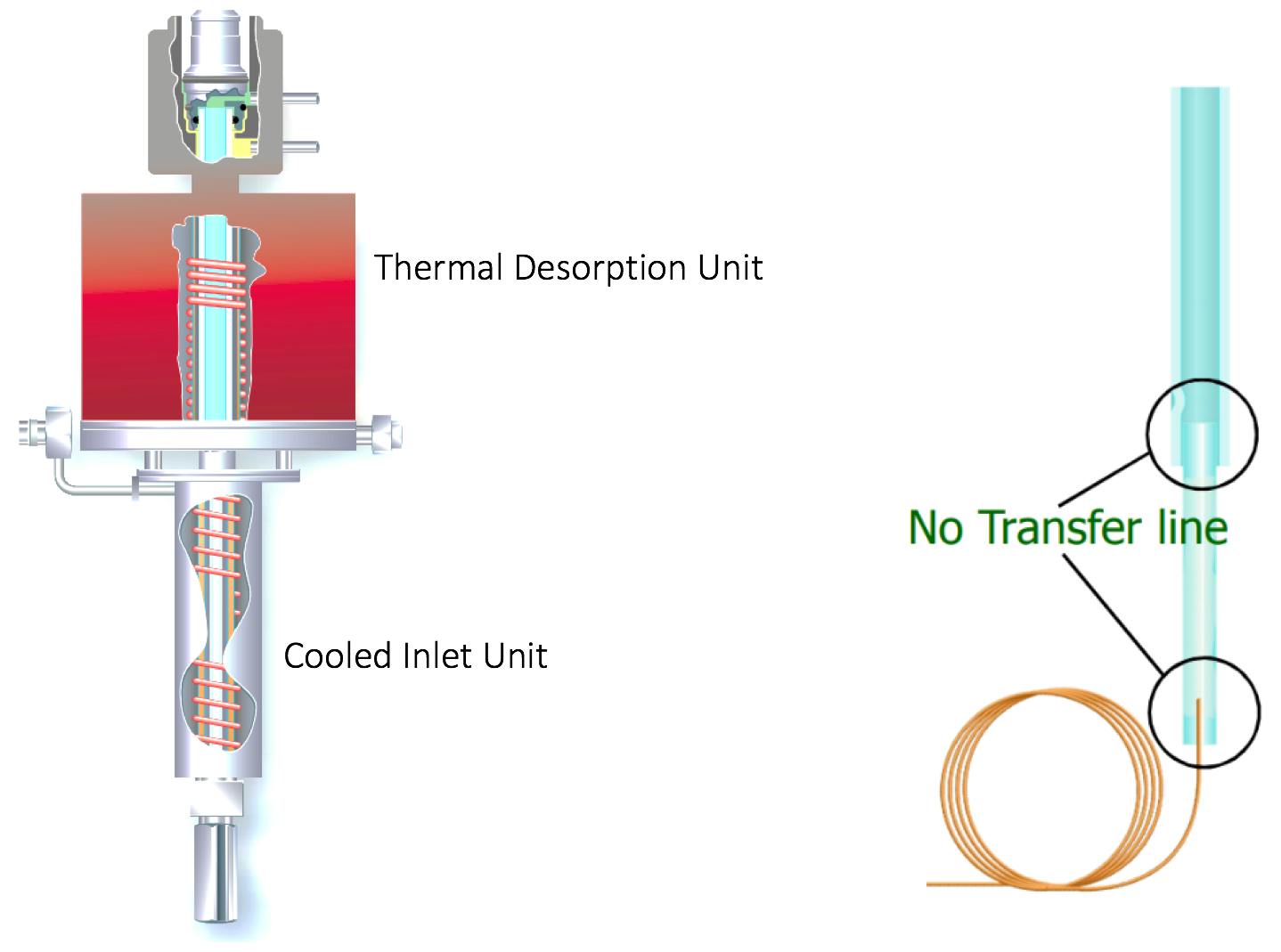
Figure 3: Schematic of an automated dynamic headspace extraction / cooled inlet system combination (the cooled inlet may be Peltier or cryogenically cooled) with ‘zero transfer line’ configuration – leading to zero loss in transfer from the thermal desorption tube to the GC column
Figure 4 shows the results of a comparative study of headspace techniques for a sample of dry tea [1] which highlights two advantages of the basic dynamic headspace technique. It is able to deal with solid samples (1g of dry tea) and, as can be seen, it is more comprehensive and sensitive for this particular application (which is generally true) compared to static headspace sampling.
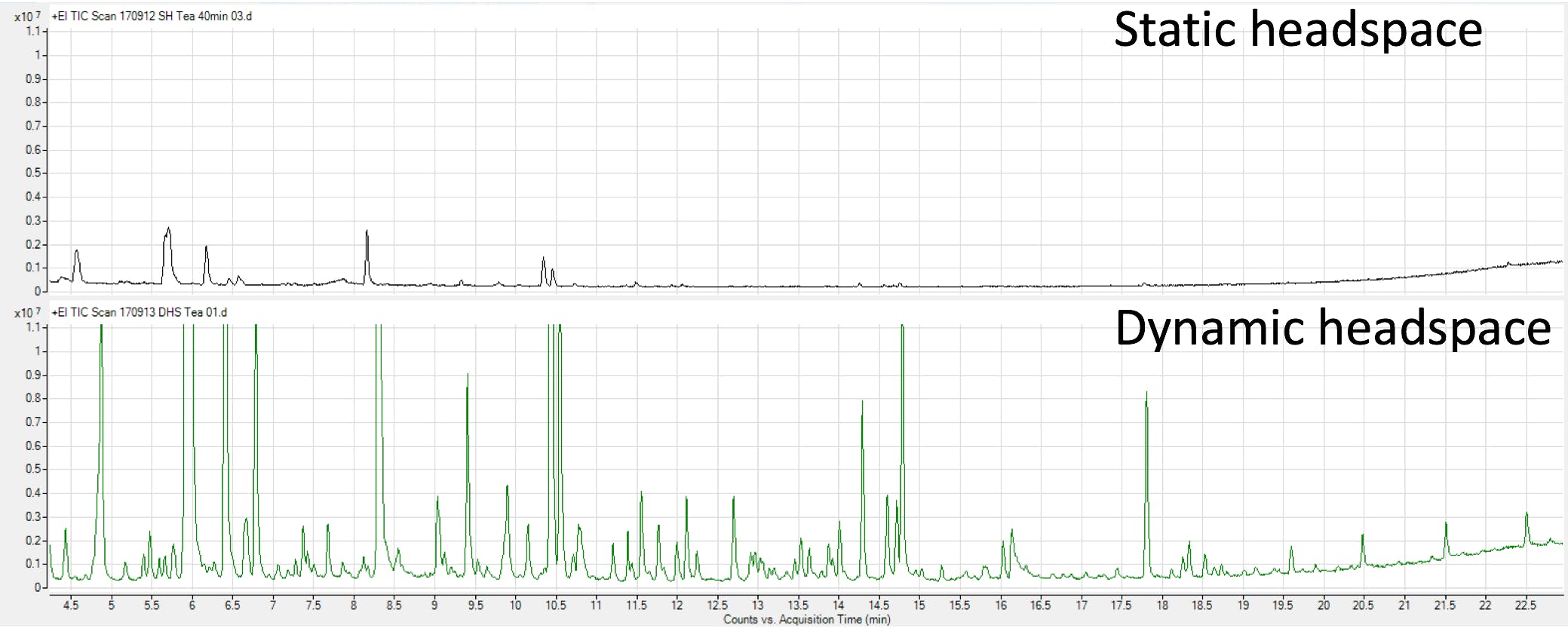
Figure 4: Chromatograms from the extraction of dry tea samples (1g) using both static and dynamic headspace sampling. For the DHS extraction, a TENAX TA trap was used and, in both cases, split injection was used with a 10:1 without cryo-cooling. (Reproduced with permission from Ref. 1)
Full Evaporative Technique (FET)
An adaptation of any headspace sampling technique is known as the Full Evaporative (Evaporation) Technique (FET). In this technique, a relatively small volume of sample is used (typically <100μL) with a headspace vial of 10 or 20mL volume, in which the sample (including the sample matrix) is fully evaporated. This approach can be very useful when the volatile and semi-volatile analytes have higher distribution constants indicating a preference to remain within the sample matrix or diluent, rather than volatilise into the headspace. This is typical when, for example, the sample is aqueous, and the analytes are polar, meaning they have a high chemical affinity for the sample matrix. Or, where analytes have a low vapour pressure even under elevated extraction temperatures or increased agitation.
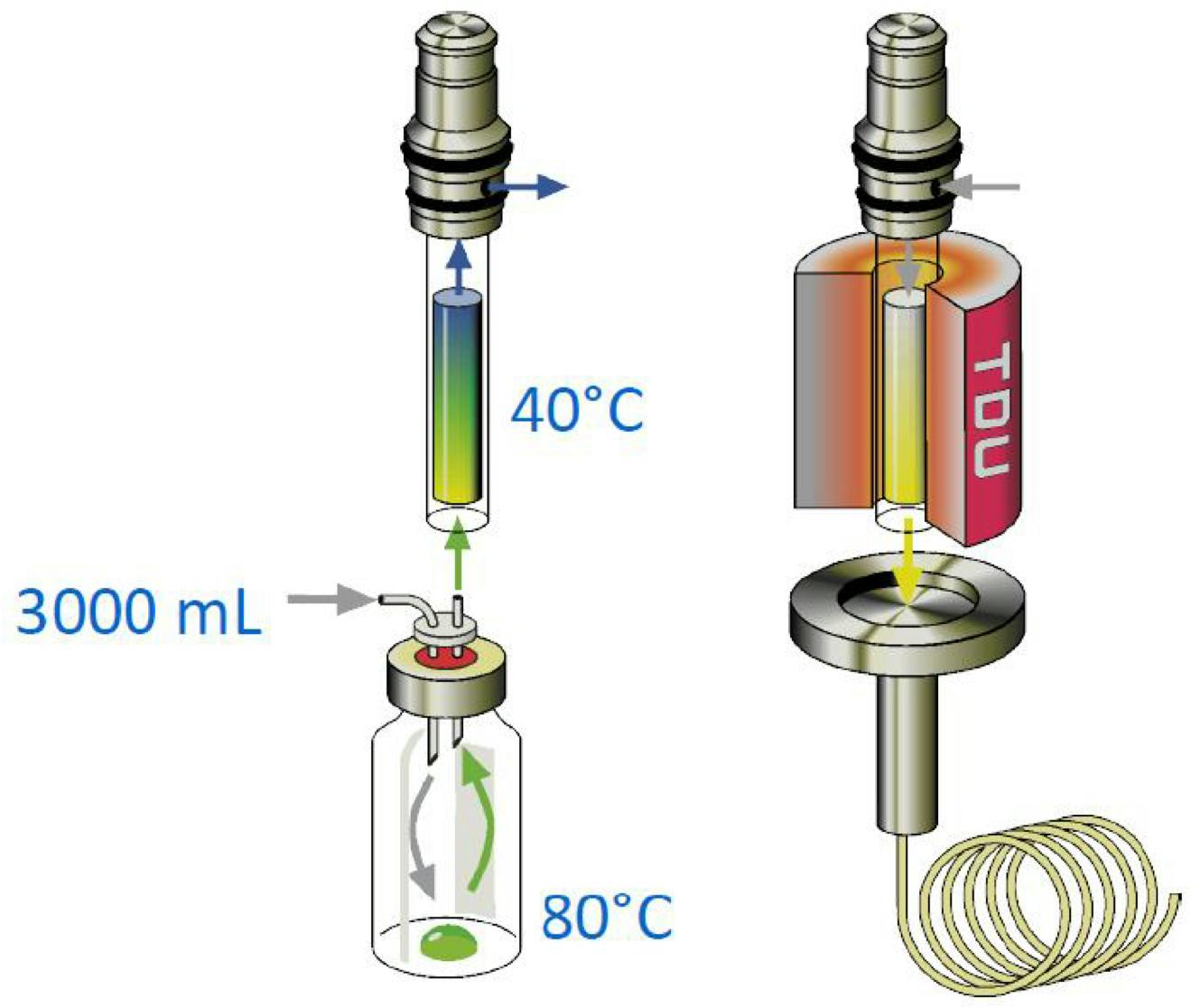
Figure 5: Schematic representation of an automated dynamic headspace extraction using the Full Evaporation Technique (FET) (Courtesy of Gerstel GmbH)
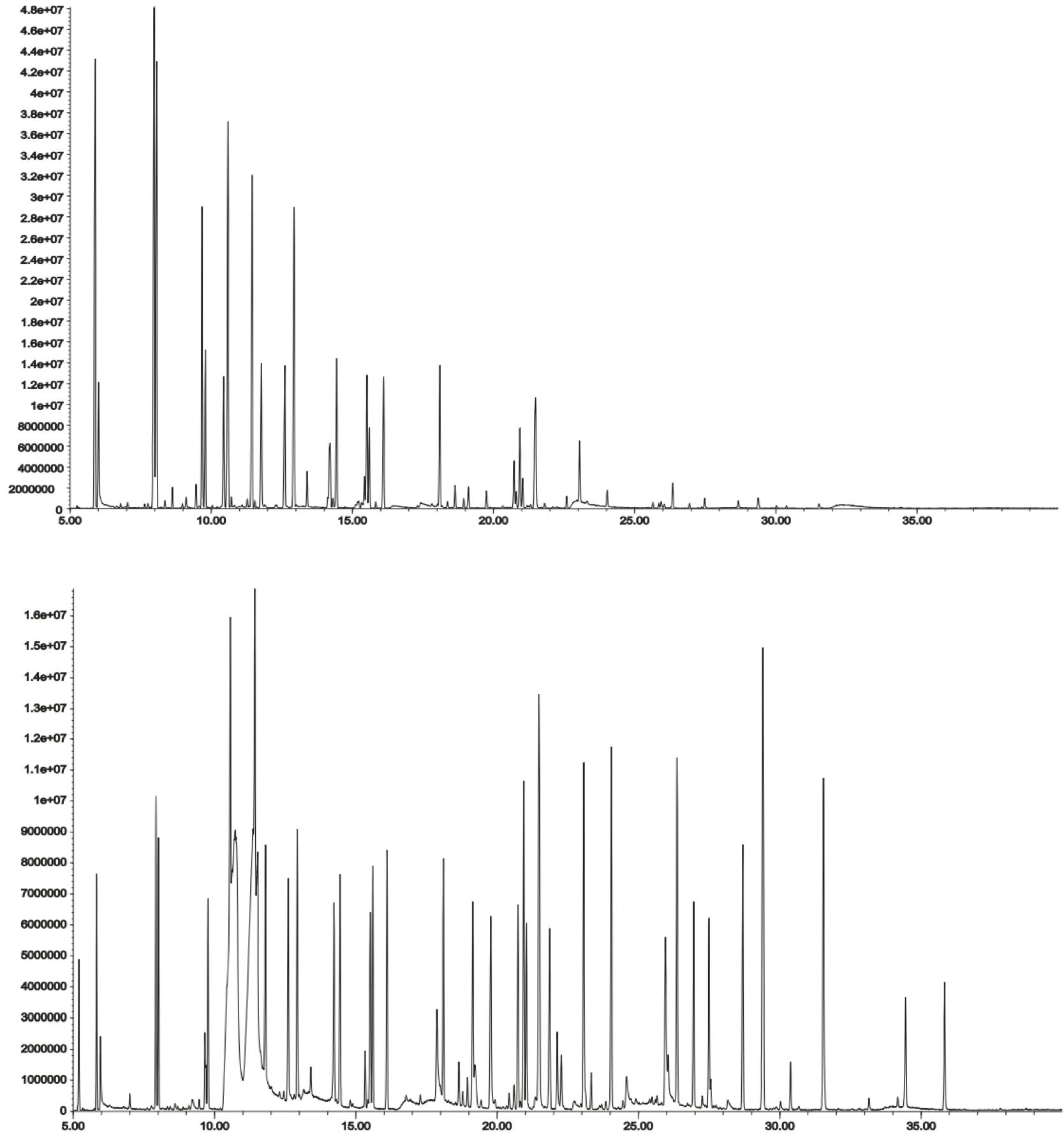
Figure 6: DHS chromatogram of 2 g of spiked shampoo (top), FET-DHS analysis of a 20 μL sample of spiked shampoo (diluted 1:9 in methanol)(bottom). GC Column 5% phenyl polydimethylsiloxane. [2]
What should be immediately obvious from Figure 5 is the FET-DHS sample extract bias towards an increase in compounds at later elution times, which should represent higher boiling or more polar compounds. This correlates with analytes whose distribution constants are higher. The FET technique obviously presents a better option where analytes are more intractable.
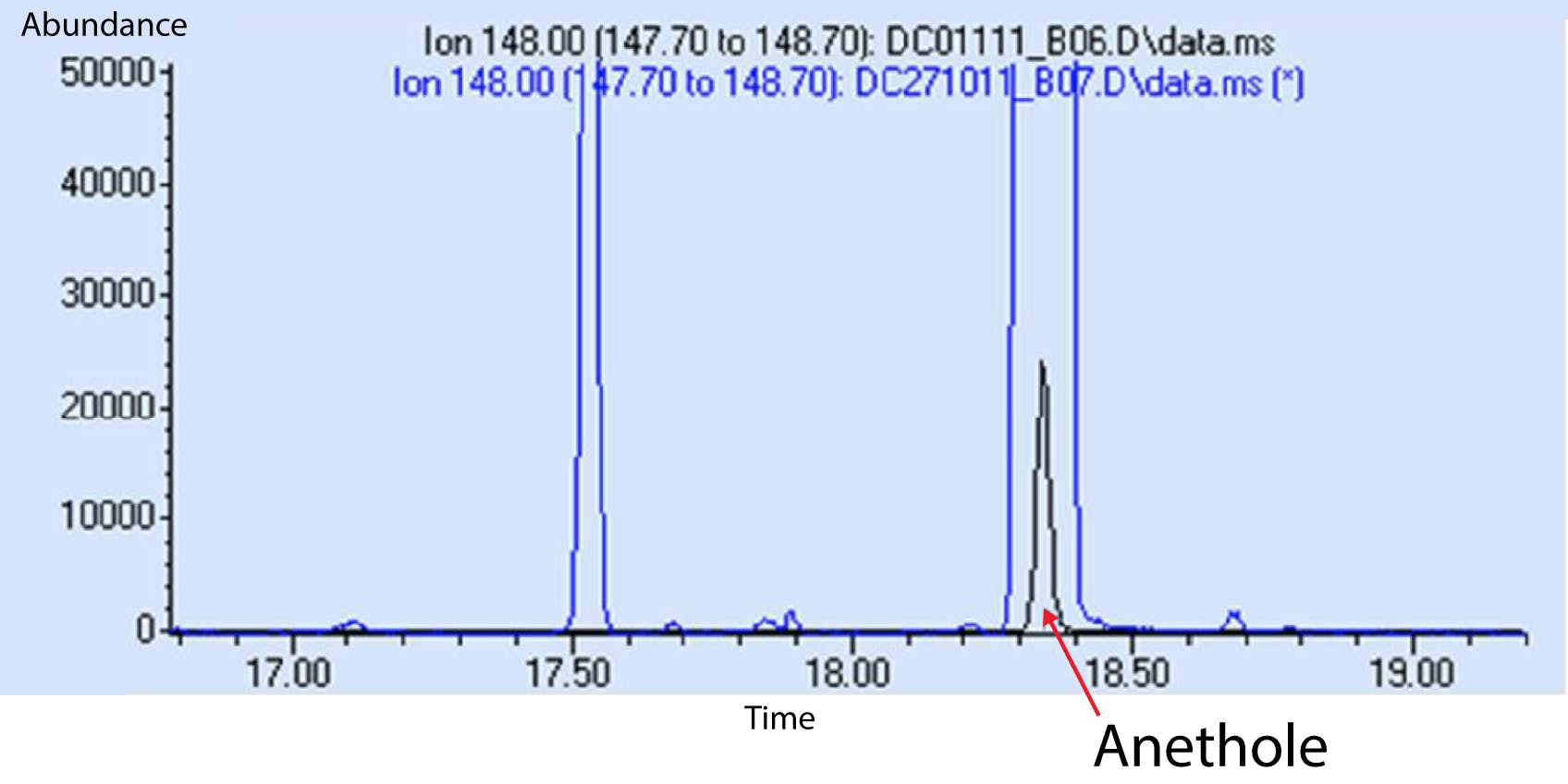
Figure 7: Chromatogram of static headspace sample (black), and FET-DHS (blue) for a sample of herbal liquor. [Reproduced with permission Anatune, Cambridge, UK]
Figure 7 also highlights the further advantage of the FET technique when undertaking quantitative targeted analysis where one can clearly see the sensitivity differences between the two extraction techniques [3].
Multi Volatiles Method (MVM)
A further, very interesting technique, is the Multi Volatiles Method (MVM) which represents an excellent way to ensure that all volatile compounds, or indeed all analytes of interest, are identified and quantified, even within highly complex matrices. Using this approach, the full evaporation technique can be used with sequential dynamic headspace extractions under different temperature and flow conditions and using different thermal desorption trap materials. The idea is to have a comprehensive headspace extraction technique which is capable of extracting analytes with a wide range of differing chemistries and, resultantly, distribution constants. This process is shown in schematic form in Figure 8.
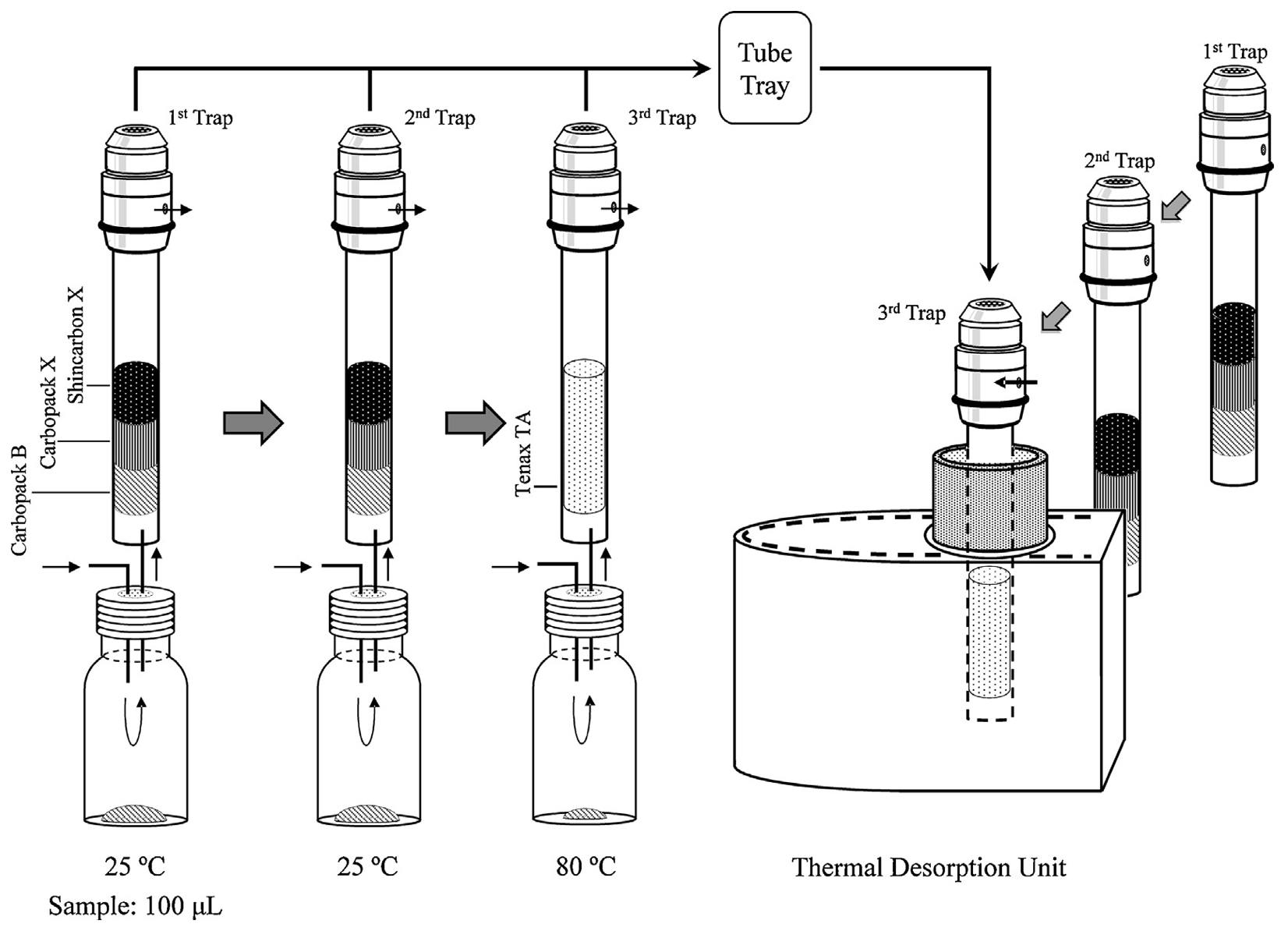
Figure 8: Schematic procedure for multi-volatile method (MVM) analysis with sequential DHS sampling [4]
Using the MVM approach, each of the thermal desorption tubes may be analysed separately to ‘fractionate’ the headspace out. Alternatively, when combined with a cryo-inlet, each tube may be desorbed into the inlet and the total output can then be introduced onto the GC column for a ‘full profile’ type approach. This represents an approach which is much more comprehensive than static headspace GC. Reference 5 outlines various approaches to quantitative analysis using the technique.
The desorption tube materials can be selected to match the analyte chemistries associated with each ‘fraction’; however, in most applications I have seen, these tend to be restricted to three different packing varieties.
As the process can be automated, even a full experimental design approach can be taken to optimise various experimental parameters in an overnight campaign.
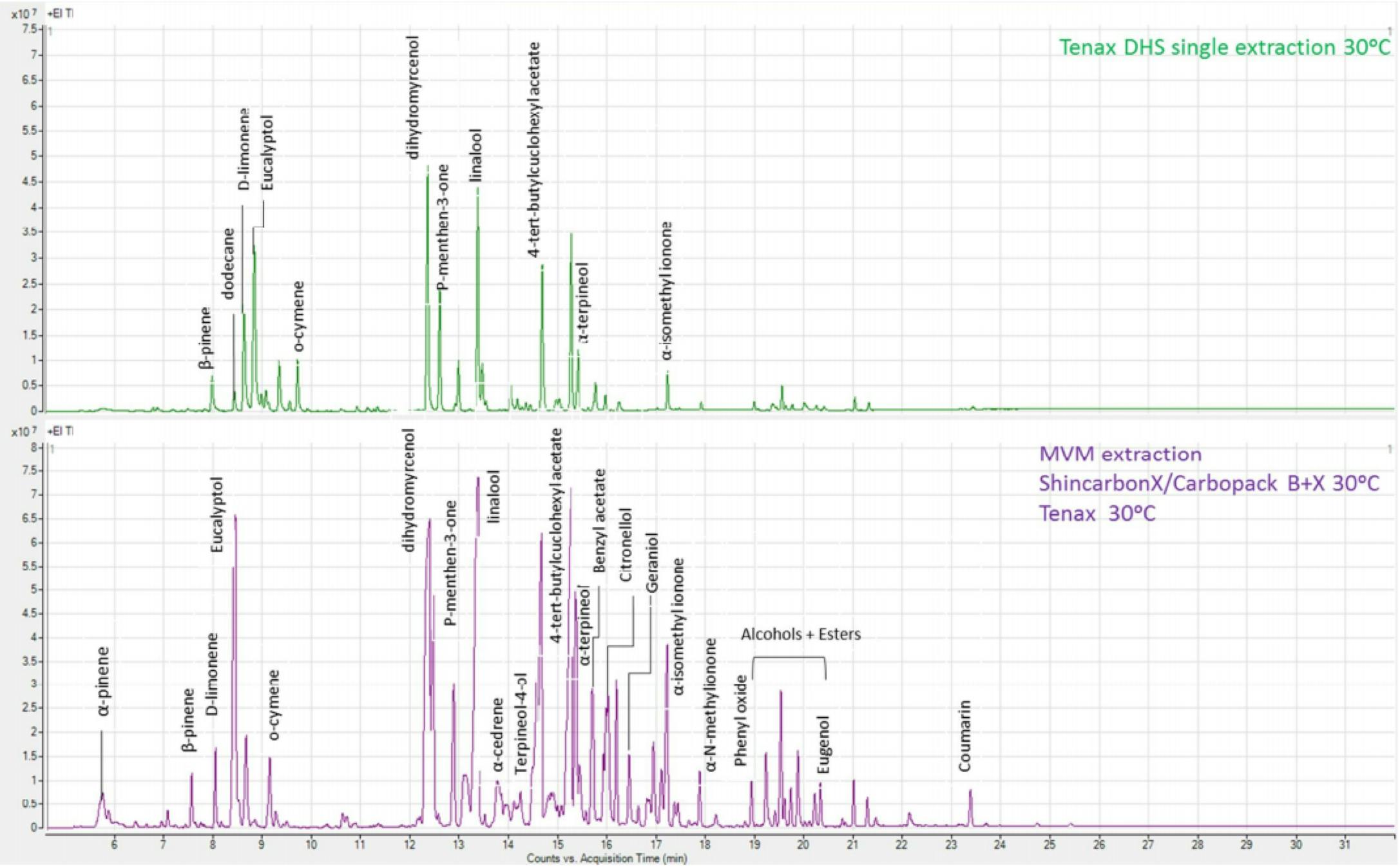
Figure 9: Comparison of aroma extractants from a soap sample using a single-stage DHS approach (top) and a two-stage FET Multi Volatiles Method approach (bottom) [Reproduced with permission Anatune, Cambridge, UK]
Figure 9 highlights the comprehensive nature of the data obtained from the FET-MVM approach.
Are These Techniques Worth The Trouble?
At this point, work continues on a number of these headspace sampling variants in order to identify those which are most suited to our various analytical challenges. The complexity and number of variables clearly create opposition. However, the DHS-FET and DHS-MVM approaches, the ability to fully automate the development process, the comprehensive nature of the technique, the ability to deal with various matrices and the high sensitivity make them very appealing.
* Just a word on salting out to improve the static headspace recovery of polar analytes from polar matrices. We discovered a very helpful table which describes the relative efficiency of salting out in reference 6 which led us to achieve higher static headspace extraction with a much lower quantity of ammonium sulphate than the sodium chloride that we would have typically chosen.
** We are currently also investigating the use of co-solvents, higher boiling point diluents and other additives which promote analyte partitioning into the headspace, which can be very useful when dealing with non-polar matrices. For more information see references 7 and 8.
[1] Comparison of extraction techniques for volatiles in a selection of tea samples, Technical note no. AS188, Anatune, Cambridge UK.
[2] Flavor and Fragrance Analysis of Consumer Products – Dynamic Headspace Compared to Some Traditional Analysis Approaches, AppNote 6/2012, Gerstel GmbH & Co. KG, Eberhard-Gerstel-Platz 1, D-45473 Mülheim an der Ruhr, Germany
[3] Dynamic headspace (DHS) analysis using Full Evaporation Technique (FET) to quantify trace level analytes present in a herbal based liquor, Chromatography Technical Note No AS106, Dan Carrier, Anatune Ltd. Cambrige, UK.
[4] Multi-volatile method for aroma analysis using sequential dynamic headspace sampling with an application to brewed coffee, Nobuo Ochiai, JunTsunokawa, Kikuo Sasamoto, Andreas Hoffmann, Journal of Chromatography A, Volume 1371, 5 December 2014, Pages 65-73
[5] Extension of a dynamic headspace multi-volatile method to milliliter injection volumes with full: sample evaporation: Application to green tea, Nobuo Ochiai, JunTsunokawa, Kikuo Sasamoto, Andreas Hoffmann, Kazunori Okanoya, Kevin MacNamara, Journal of Chromatography A, Volume 1421, 20 November 2014, Pages 103-113
[6] Room temperature ionic liquid as matrix medium for the determination of residual solvents in pharmaceuticals by static headspace gas chromatography, Liu FH, Jiang Y J Chromatogr A 2007 1167: 116-119.
[7] Utilization of a Matrix Effect to Enhance the Sensitivity of Residual Solvents in Static Headspace Gas Chromatography, Zhi Chen et. al. Chen et al., J Chromatogr Sep Tech 2015, 6:6